Research Article - Neuropsychiatry (2017) Volume 7, Issue 2
The effect of ketamine on microglia and proinflammatory cytokines in the hippocampus of depression-like rat
- Corresponding Author:
- Xiaobin Wang M.D
Department of Anesthesiology, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan Province, China;
Phone: 0830-3165500
Fax: 0830-3165500
E-mail: [email protected]
Abstract
Objectives
Mounting evidence suggests that activation of microglia and inflammatory response play an important role in the pathogenesis of depression. A single or repeated sub-anesthetic dose of ketamine administration induced fast and potent antidepressant effect. We investigated the effect of ketamine on microglia activation and pro-inflammatory cytokines levels in hippocampus of depression-like rats.
Methods
Forty-eight rats were equally randomized into four groups Control+saline group, Control+ketamine group, Chronic unpredictable mild stress (CUMS)+saline group and CUMS+ketamine group. 0.9% saline or 10 mg/kg ketamine was given once daily for 7 consecutive days. The sucrose preference test (SPT) open field test (OFT) and forced swimming test (FST) were performed before and day 7 after drug treatment. The hippocampus was subsequently harvested for the detection of levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6 and levels of CD11b and Iba1. The CD11bpositive cells in the CA3 and DG region of the hippocampus were also stained by immunohistochemistry.
Results
CUMS induced the decrease of sucrose solution intake volume, the increase of the immobility time, the up-regulation of TNF-α, IL-1β, IL-6, CD11b and Iba1 levels of the hippocampus and the increase of CD11b-positive microglia in the CA3 and DG region of the hippocampus. Ketamine administration could reverse this effect.
Conclusion
Ketamine’s antidepressant effect on depression-like rats is accompanied by the inhibition of microglia activation and pro-inflammatory cytokines levels in the hippocampus.
Keywords
Depression, Ketamine, Microglia, Pro-inflammatory cytokines
Introduction
Depression is a common clinical mental disorder [1], it is estimated that depression will be the second global burden of disease after ischemic heart disease by 2020 [2]. Traditional antidepressants need several weeks to produce antidepressant effect, however, risk of suicide increases during this time [3]. Therefore, there is an emergency need to exploit novel antidepressants with fast-acting and high remission rate.
Recent studies have shown the antidepressant effect of ketamine, acting as a nonselective N-methyl-D-aspartate (NMDA) receptor antagonist [4]. A single sub-anesthetic dose of ketamine administration displayed rapid and obvious antidepressant properties, but the antidepressant effect of ketamine just maintains a short time [4,5]. It has been demonstrated that repeated ketamine administration can maintain ketamine’s antidepressant effects [6]. Ketamine can inhibit microglia activation stimulated by lipopolysaccharide (LPS) and pulmonary inflammatory responses induced by sepsis [7,8]. However, whether the microglia deactivation and anti-inflammatory cytokines mediate ketamine’s antidepressant effect is unclear. We hypothesize that the microglia deactivation and down-regulation of pro-inflammatory cytokines may be one of the possible mechanisms underlying ketamine’s antidepressant effects. Thus, we try to investigate the effect of ketamine on the inhibition of microglia activation and proinflammatory cytokines in the CUMS rats.
Materials and methods
▪ Animals
Forty-eight male adult Sprague-Dawley rats (age of 50 days, weight of 200-250 g) were purchased from the Southwest Medical University Animal Centre. They were housed three per cage with food and water available ad libitum. The rats were maintained on a 12-h light/dark cycle (lights on at 7.00 a.m.) at a temperature of 23℃ ± 1℃. They were left to acclimate for 7 days before subjected to CUMS. All experimental procedures were approved by the Animal Ethics Committee of Southwest Medical University and were performed in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health, USA.
▪ CUMS and drug administration
The CUMS procedure was carried out according to previous reports [9]. The rats in CUMS group were exposed to 9 stressors with 1 time per day. The CUMS procedure sustained 6 weeks and the same stressor was not applied consecutively over two days to prevent rats from tolerance to the stressors. The stressors included: (1) cage tilting 45°C for 24 h, (2) wet bedding for 24 h (200 ml water to wet bed ), (3) hot swimming at 45℃ for 5 min, (4) cold swimming at 4℃ for 5 min, (5) fasting for 24 h, (6) water deprivation for 24 h, (7) rotation for 60 min (at 30 revolutions per minute), (8) tail nip for 1 min (1 cm from the end of the tail), and (9) inversion of light/dark cycle for 24 h. The rats in control group were fed in a quiet room for 6 weeks except necessary procedures, such as routine cage cleaning.
After 6 weeks, 24 Control rats were equally randomized into Control+saline group and Control+ketamine group, 24 CUMS rats were equally randomized into CUMS+saline group and CUMS+ketamine group. 0.9% saline or 10 mg/kg ketamine was intraperitoneally injected once daily for 7 consecutive days [10].
▪ Behavioral tests and tissue preparation
The behavioral tests were conducted after CUMS model and 7 days after drug administration.
Sucrose preference test
The anhedonia is the core symptom of depression, and it is reflected by the percentage of sucrose solution consumption. The rats were habituated two bottles of 1% sucrose solution for 24 h. The one bottle of sucrose solution was changed into water at the second day. The rats were allowed to drink 1% sucrose solution (100 ml) and the same volume of water for 3 h after fasting and water deprivation 24 h. Sucrose preference was defined as the percentage of sucrose solution consumption of the total fluid volume during 3 h.
Open-field test
The open field consists of a blue arena of 100 cm × 100 cm square floor surrounded by 50 cm high walls. The floor of the apparatus was divided into 25 equal squares (20 cm × 20 cm each) by white lines. Rats were gently placed into the center of floor, and the number of crossing and rearing activity performed by each rat was recorded by an expert observer for 5 minute.
Forced swimming test (FST)
The forced swimming test was conducted in a cylinder with 65 cm tall, 30 cm in diameter and filled with 40 cm-depth of water (22-23°). The FST was performed for 6 min, and the immobility time (in seconds) was recorded during the final 5min. The immobility time was defined as the time during which the rat stood still without struggling or used only essential movements to keep the head above water or contact the bottom more than 1 second.
After the final behavioral test, the rats were anesthetized with sodium pentobarbital and sacrificed. The skulls were removed and hippocampus was dissected after perfused with 4% paraformaldehyde and then stored in 70% alcohol until embedded with paraffin.
▪ Enzyme-linked immunosorbent assay (ELISA)
TNF-α, IL-1β and IL-6 levels in hippocampus were measured using ELISA-kit (Shanghai MLBIO Biotechnology, China). Briefly, rat hippocampus tissue was homogenized in iced RIPA buffer with PMSF, and protein concentration was measured by BCA protein assay (Bioworld Technology, USA), then according to the manufacturer’s instructions operation, concentrations of TNF-α, IL-1β and IL-6 were calculated by referring to a standard curve.
▪Western blotting
CD11b and Iba1 levels in hippocampus were measured by western blot analysis. The hippocampus tissue was homogenized in iced RIPA buffer with PMSF, and then protein concentration was measured by BCA protein assay (Bioworld Technology, USA). Protein samples were separated on 10% or 15% SDS-PAGE and transferred to polyvinylidene fluoride membrane (PVDF). Membranes were blocked with 5% non-fat milk for 1h and incubated with primary antibodies [CD11b(1:200, Santa Cruz, Iba1 (1:1000, ProteinTech Group, Chicago, USA, GAPDH (1:3000; ProteinTech Group, Chicago, USA)] at 4°C overnight. The membranes were washed with TBST buffer and incubated with secondary antibody (1:5000)at room temperature for 1 h. The intensity of the protein bands was quantified using Image J software.
Immunohistochemistry (IHC)
CD11b-positive cells in the CA3 and DG region of the hippocampus were measured by immunohistochemistry. The rat hippocampus was embedded in paraffin, and the paraffin blocks were sectioned at 5μm. The sections were deparaffinized, ethanol hydrated, and submitted to heat-induced antigen retrieval. 3% hydrogen peroxide was used to quench endogenous peroxidases, and the sections were rinsed with PBS, and blocked with 10 % norm goat serum, and subsequently incubated with the primary antibodies of CD11b (Santa Cruz, USA), and then incubated with the corresponding goat antimouse secondary antibody (Santa Cruz, USA). 3,3-diaminobenzidine (DAB) was used for the chromogenic reaction. Finally, the sections were dehydrated, cleared, and coverslipped. Images were captured with OLYMPUS digital imaging system (40×). The immunopositive cells were counted within 5-image (1360 × 1024 pixels) of the hippocampal CA3 and DG region.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) 16.0 and GrphPad Prism 5.0 were used for statistical analysis. All data were presented as mean ± SEM. Differences between two groups were determined by Student’s t-test and multigroup were determined by oneway ANOVA followed by Tukey’s post hoc test. p values less than 0.05 were considered as statistically significant differences.
Results
▪ Effects of CUMS exposure and ketamine treatment on the behavioral indexes
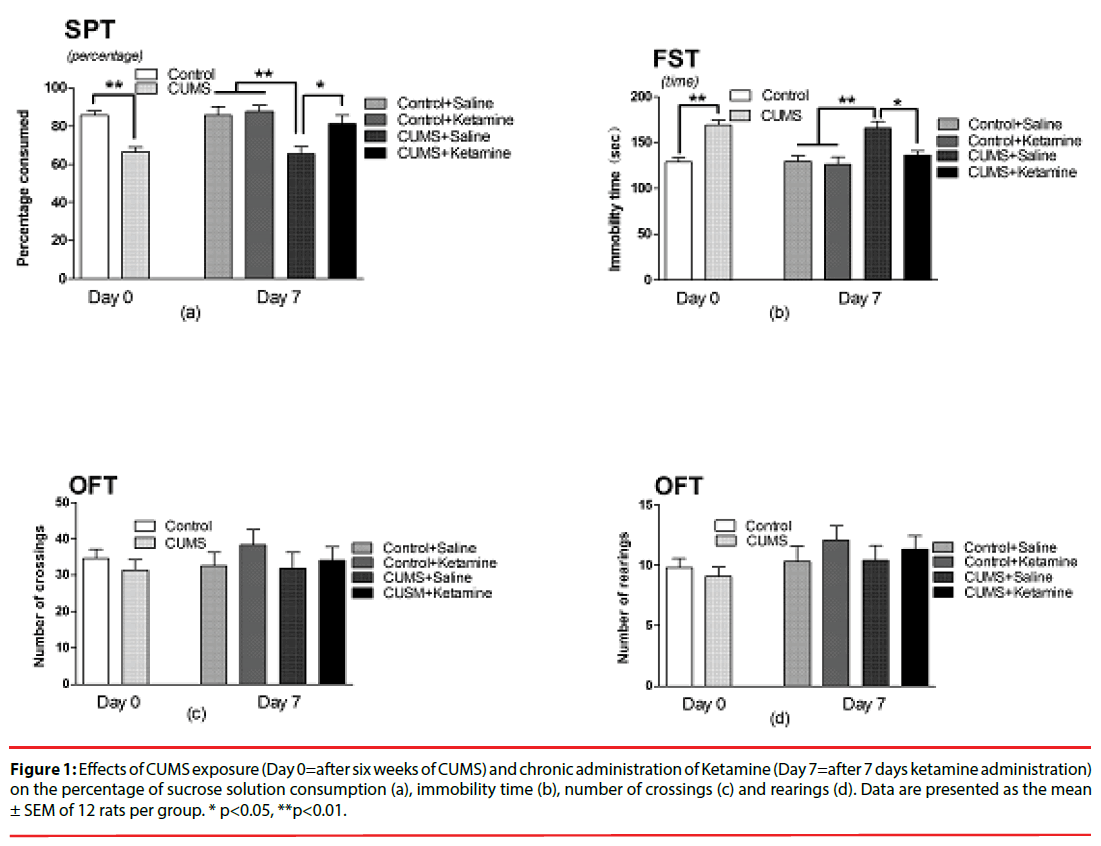
As depicted in Figure 1, compared with the control, rats in CUMS group displayed the significant decrease of 1% sucrose solution intake volume (t=5.624, P<0.001, Figure 1a) and the increase of immobility time in FST (t=5.597, P<0.001, Figure 1b). Compared with the CUMS+Saline group, rats in CUMS +Ketamine group displayed the significant increase of 1% sucrose solution intake volume (F=6.522, P=0.0014, Figure 1a) and the decrease of immobility time in FST (F=5.624, P=0.01, Figure 1b). However, CUMS exposure and ketamine administration did not affect the number of crossings (t=0.7908, P=0.4346; F=0.6773, P= 0.5114; Figure 1c) and rearings (t=0.6700, P =0.5030; F= 0.4896, P= 0.6920; Figure 1d) in OFT.
Figure 1: Effects of CUMS exposure (Day 0=after six weeks of CUMS) and chronic administration of Ketamine (Day 7=after 7 days ketamine administration) on the percentage of sucrose solution consumption (a), immobility time (b), number of crossings (c) and rearings (d). Data are presented as the mean ± SEM of 12 rats per group. * p<0.05, **p<0.01.
▪ Effects of ketamine on the levels of TNF- α,IL-1β and IL-6 in the hippocampus
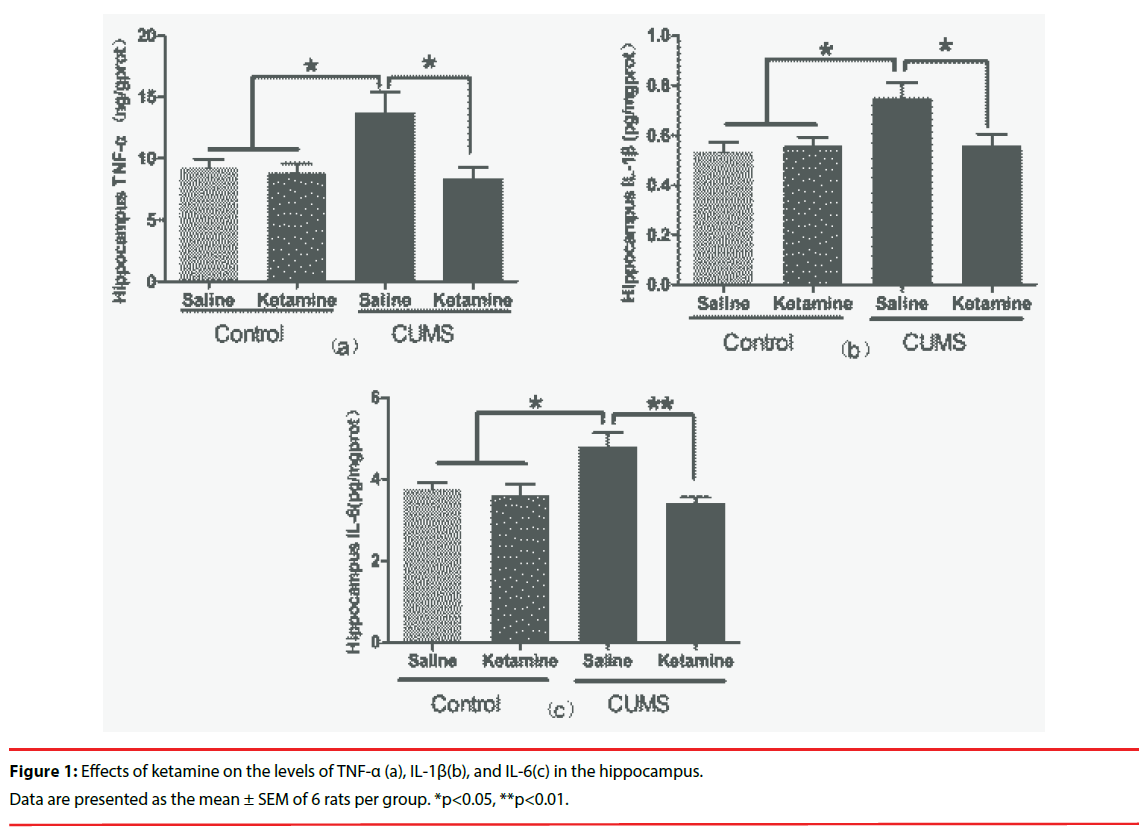
As depicted in Figure 2, compared with the control group, the levels of TNF-α, IL- 1β and IL-6 were significantly increased in the CUMS rat hippocampus (F=5.330, P=0.0073, Figure 2a; F=4.736, P=0.0118, Figure 2b;F=6.725,P=0.0026, Figure 2c). Interestingly, repeated ketamine treatment significantly attenuated the increase of TNF- α,IL-1β, and IL-6 levels in the hippocampus of CUMS rats (Figure 2a, Figure 2b and Figure 2c)].
▪ Effects of ketamine on the levels of CD11b and Iba1 in the hippocampus
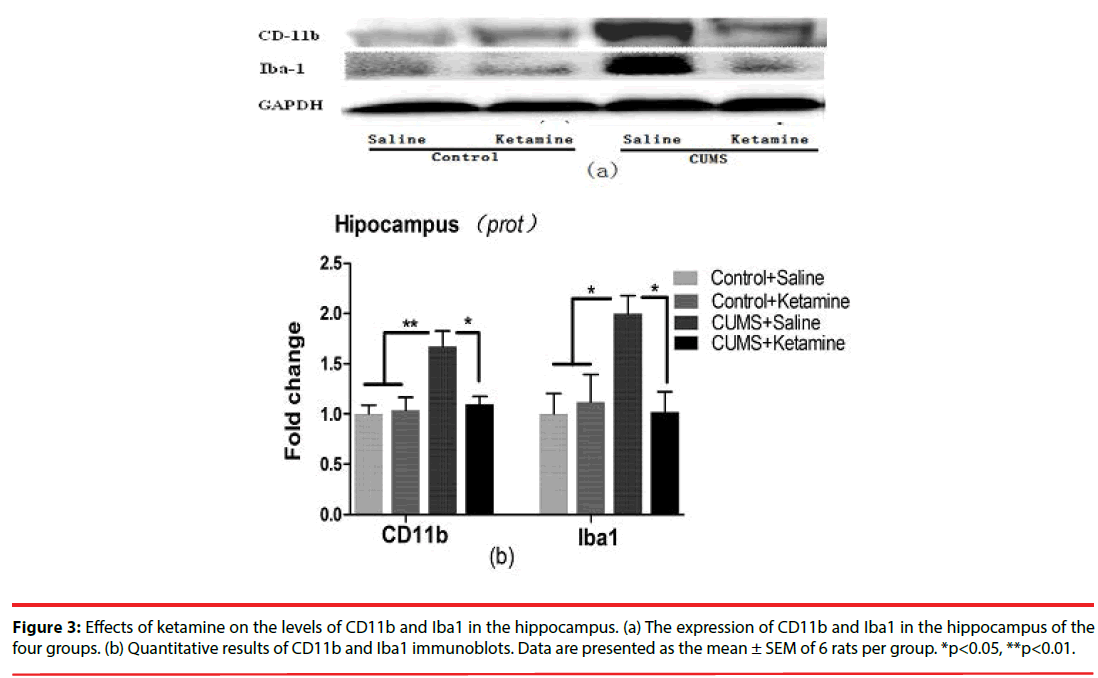
As depicted in Figure 3, compared with the control group, the levels of CD11b (F=7.411, P=0.0016, Figure 3b) and Iba1 (F=4.735, P=0.0118, Figure 3b) were significantly increased in the hippocampus of CUMS rat. Moreover, repeated ketamine treatment reversed the increase of CD11b and Iba1 levels in the hippocampus of CUMS rats (Figure 3b).
▪ Effects of ketamine on CD11b-positive cells in the CA3 and DG region of the hippocampus
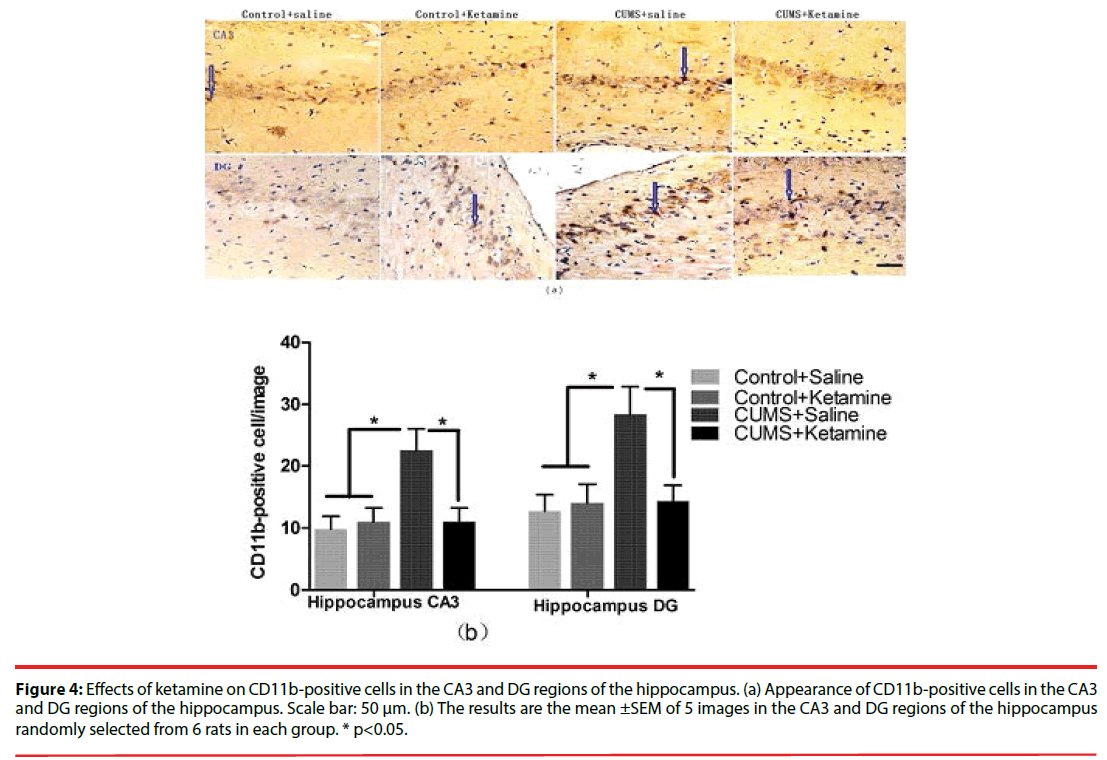
As depicted in Figure 4, compared with the control, the CD11b-positive cells were significantly increased in the CA3 (F=5.213, P=0.0080, Figure 4b) and DG (F=4.914, P=0.0102, Figure 4b) regions of the hippocampus in the CUMS rat. The increase of CD11b-positive cells in CA3 and DG regions of the hippocampus were reversed by repeated ketamine treatment (Figure 4b).
Figure 4: Effects of ketamine on CD11b-positive cells in the CA3 and DG regions of the hippocampus. (a) Appearance of CD11b-positive cells in the CA3 and DG regions of the hippocampus. Scale bar: 50 μm. (b) The results are the mean ±SEM of 5 images in the CA3 and DG regions of the hippocampus randomly selected from 6 rats in each group. * p<0.05.
Discussion
Recently, preclinical and clinical studies have demonstrated that ketamine have fast and obvious antidepressant effects [11], but the mechanisms are still unclear. Relative studies reveal that neuroinflammation may contribute to depression etiology [12]. As is well-known, the microglia plays a key role in inflammation response of CNS [13]. And several researches have reported that ketamine could inhibit inflammatory cytokine release in systemic inflammatory response syndrome [14,15]. Thus, we hypothesized that ketamine’s antidepressant effects may be related to its anti-inflammation action in CNS. We investigated the effect of ketamine on microglia activation and proinflammatory cytokines release in CNS on depression-like animal model.
There are limitations in methodology and ethics on the human central nervous system. Therefore, the establishment of animal model about CNS disorders is widely used for further research [16,17]. CUMS model is widely used in depression research [18]. So in the present study, we chose CUMS procedure to establish the depression-like rat model. Clinical stressrelated depressive patients often have obvious depressive mood. Many behavior tests, such as the percentage of sugar solution consumption (which reflects the emotional activities about interest or anhedonia) and immobility time in FST (which reflects desperate state) were used to evaluate the depression-like state of CUMS rats. There was no difference between control and CUMS at the baseline of behavioral test before the CUMS exposure, but after the CUMS, compared with Control, rats in CUMS significantly displayed the decrease of sucrose solution intake volume and the increase of immobility time. Our results also showed that the CUMS model was successfully established.
Several researches have shown that inflammatory processes were involved in the pathogenesis of major depression [19,20]. Interestingly, the pharmacological antidepressant treatment was associated with the reduction of inflammatory cytokines levels and depressive symptoms [21]. Dowlati et al. found the proinflammatory cytokines of TNF-α and IL-6 in depressive patients were significantly higher than that in normal subjects [22]. Maes et al. have reported that psychological stress increased the levels of TNF-α, IL-6, IL-1 receptor antagonist (IL-1Ra), interferon gamma (IFN-gamma) and IL-10 in subjects [23]. Animal researches also supported that stress could increase the levels of inflammation cytokines such as TNF-α and IL-6 in periphery or the central nervous system [24,25]. In the present study, we also demonstrated that CUMS exposure increased the levels of TNF-α, IL-6 and IL-1β in hippocampus.
Previous studies have shown that active microglia played an important role in the neuroinflammation response because the active microglia may lead to the upregulation of CD11b and Iba1, and produce neurotoxity and pro-inflammatory cytokines [26,27]. Postmortem study results provided the evidences that microglia was activated in the anterior cingulate cortex, frontal cortex and hippocampus of patients with depression [28,29]. Therefore, active microglia and levels of pro-inflammatory cytokines may be used as predictors to evaluate antidepressant treatment [30,31]. Chronic stress was associated with microglia activation and morphological change [32,33]. Our study also showed that the CUMS exposure induced microglia activation in hippocampus (up-regulation of protein CD11b and Iba1).
A single sub-anesthetic dose of ketamine administration showed rapid and obvious antidepressant properties, but just maintained a short time [4,5]. Graeme et al. have reported sub-anesthetic dose of ketamine administration for 5 consecutive days relieved patients’ depressive symptoms quickly and maintained several weeks [34]. Garcia et al. have demonstrated that repeated ketamine administration also produced antidepressant effect [35]. In our study, sub-anesthetic dose of ketamine administration for 7 consecutive days showed remarkable relief of behavioral changes induced by CUMS exposure, and was associated with the inhibition of microglia and pro-inflammatory cytokines in hippocampus, and it has been reported that ketamine may decrease the levels of inflammation cytokines in sepsis patients [36], inhibit the activation of microglia and the levels of inflammation cytokines in cells induced by lipopolysaccharide (LPS) [7,8]. The mechanism may be that the proinflammation cytokines induced indoleamine 2, 3-dioxygenase (IDO) activation (the limited enzyme in kynurenine pathway), further activated kynurenine pathway to produce neuroactive metabolites and disturb the homeostasis between monoamine and glutamate neurotransmitter, which plays a key role in the pathogenesis of depression [37-39]. In addition, the pro-inflammation cytokines also promoted release of adrenocortical hormone to activate the hypothalamic pituitary gonadal axis (HPA), hyperactive HPA may induce hypercortisolemia and elicit symptoms of depression [40,41]. Therefore, these results showed that ketamine’s antidepressant effects were associated with the inhibition of microglia activation and pro-inflammatory cytokines in the CUMS rats.
In conclusion, CUMS exposure elicited the depression-like symptoms and was associated with the microglia activation and the increase of pro-inflammatory cytokines levels in the hippocampus. The repeated ketamine administration relieved the depressionlike symptoms and was associated with the inhibition of microglia activation and pro-inflammatory cytokines levels in the hippocampus. The anti-inflammatory effect of ketamine in CNS may be one of the possible mechanisms underlying ketamine’s antidepressant effects.
Acknowledgments
This work was supported by the Projects of the National Natural Science Foundation of China (Grant No. 81271478), Department of Science &Technology of Sichuan Province (Grant No. 14JC0093), Department of Education of Sichuan Province (Grant No. 14ZA0142) and Department of Science &Technology of Luzhou City (Grant No. 2015-S-46) to X. Wang.
Conflict of interest
The authors have no potential conflicts of interest to disclose.
References
- Kuo DC, Tran M, Shah AA, et al.Depression and the Suicidal Patient. Emerg. Med. Clin. North Am 33(4),765-778(2015).
- Li Z, Li Y, Chen L, et al.Prevalence of Depression in Patients with Hypertension: A Systematic Review and Meta-Analysis. Medicine (Baltimore)94(31),e1317(2015).
- Thase ME, Haight BR, Richard N, et al.Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J. Clin. Psychiatry66(8),974-981(2005).
- Berman RM, Cappiello A, Anand A, et al.Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry47(4),351-354(2000).
- Zarate CA Jr, Singh JB, Carlson PJ, et al.A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry63(8),856-864(2006).
- Aan het Rot M, Collins KA, Murrough JW, et al.Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry67(2),139-145(2010).
- Chang Y, Lee JJ, Hsieh CY, et al.Inhibitory effects of ketamine on lipopolysaccharide-induced microglial activation. Mediators. Inflamm2009,705379(2009).
- Yu M, Shao D, Yang R, et al.Effects of ketamine on pulmonary inflammatory responses and survival in rats exposed to polymicrobial sepsis. J. Pharm. Sci10(4),434-442(2007).
- Surget A, Wang Y, Leman S, et al. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology 34(6),1363-1380(2009).
- Zhang GF, Liu WX, Qiu LL, et al.Repeated ketamine administration redeems the time lag for citalopram's antidepressant-like effects. Eur. Psychiatry30(4),504-510(2015).
- Niciu MJ, Henter ID, Luckenbaugh DA, et al.Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu. Rev. Pharmacol. Toxicol 54(1), 119-139(2014).
- Brites D, Fernandes A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell. Neurosci9(1), 476(2015).
- Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics 7(4),354-365(2010).
- Yang C, Hong T, Shen J, et al.Ketamine exerts antidepressant effects and reduces IL-1beta and IL-6 levels in rat prefrontal cortex and hippocampus. Exp. Ther. Med5(4),1093-1096(2013).
- Wang N, Yu HY, Shen XF, et al.The rapid antidepressant effect of ketamine in rats is associated with down-regulation of pro-inflammatory cytokines in the hippocampus. Ups. J. Med. Sci120(4),241-248(2015).
- Bovenkerk B, Kaldewaij F. The use of animal models in behavioural neuroscience research. Curr. Top. Behav. Neurosci19(1), 17-46(2015).
- Tania M, Khan MA, Xia K. Recent advances in animal model experimentation in autism research. Acta. Neuropsychiatr26(5),264-271 (2014).
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52(2),90-110(2005).
- Dahl J, Ormstad H, Aass HC, et al.The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 45(1), 77-86(2014).
- Rotter A, Biermann T, Stark C, et al.Changes of cytokine profiles during electroconvulsive therapy in patients with major depression. J. ECT29(3),162-169(2013).
- Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 36(12),2452-2459(2011).
- Dowlati Y, Herrmann N, Swardfager W, et al.A meta-analysis of cytokines in major depression. Biol. Psychiatry67(5),446-457(2010).
- Maes M, Song C, Lin A, et al.The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 10(4),313-318(1998).
- Liu W, Sheng H, Xu Y, et al. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behav. Brain. Res 242(1), 110-116(2013).
- Pan Y, Chen XY, Zhang QY, et al.Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain. Behav. Immun41(1), 90-100(2014).
- Steiner J, Bogerts B, Sarnyai Z, et al.Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World. J. Biol. Psychiatry13(7),482-492(2012).
- Kettenmann H, Hanisch UK, Noda M, et al.Physiology of microglia. Physiol. Rev91(2),461-553(2011).
- Steiner J, Bielau H, Brisch R, et al.Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res42(2),151-157(2008).
- Torres-Platas SG, Cruceanu C, Chen GG, et al.Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain. Behav. Immun 42(1), 50-59(2014).
- Munkholm K, Brauner JV, Kessing LV, et al.Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J. Psychiatr. Res47(9),1119-1133(2013).
- Watkins CC, Sawa A, Pomper MG. Glia and immune cell signaling in bipolar disorder: insights from neuropharmacology and molecular imaging to clinical application. Transl. Psychiatry4(1), e350(2014).
- Hinwood M, Tynan RJ, Charnley JL, et al.Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb. Cortex 23(8),1784-1797(2013).
- Wohleb ES, Hanke ML, Corona AW, et al.beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci31(17),6277-6288(2011).
- Correll GE, Futter GE. Two case studies of patients with major depressive disorder given low-dose (subanesthetic) ketamine infusions. Pain. Med7(1),92-95(2006).
- Garcia LS, Comim CM, Valvassori SS, et al.Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic. Clin. Pharmacol. Toxicol103(6),502-506(2008).
- Dale O, Somogyi AA, Li Y, et al.Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth. Analg115(4),934-943(2012).
- Clark SM, Pocivavsek A, Nicholson JD, et al.Reduced kynurenine pathway metabolism and cytokine expression in the prefrontal cortex of depressed individuals. J. Psychiatry. Neurosci41(4),150226(2016).
- Myint AM, Schwarz MJ, Muller N. The role of the kynurenine metabolism in major depression. J. Neural. Transm (Vienna) 119(2),245-251(2012).
- Dantzer R, O'Connor JC, Lawson MA, et al. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 36(3),426-436(2011).
- Euteneuer F, Schwarz MJ, Hennings A, et al.Depression, cytokines and experimental pain: evidence for sex-related association patterns. J. Affect. Disord 131(1-3),143-149(2011).
- Udina M, Moreno-Espana J, Capuron L, et al. Cytokine-induced depression: current status and novel targets for depression therapy. CNS. Neurol. Disord. Drug. Targets13(6),1066-1074(2014).