Research Article - (2018) Volume 8, Issue 3
Reduced Interhemispheric Resting-State Functional Homotopy in Obsessive-Compulsive Disorder
- Corresponding Author:
- Ping Li
Department of Psychiatry, Qiqihar Medical University, Qiqihar, Heilongjiang Province, 161006, China
Tel: +86 4522663403
Fax: +86 4522663766
Abstract
Neuroimaging studies have shown structural and functional abnormalities in parts of the cortico-striato-thalamo-cortical (CSTC) brain circuitry in patients with obsessive-compulsive disorder (OCD). However, little is known about changes in the interhemispheric functional homotopy in the resting-state in OCD. This study used a voxel-mirrored homotopic connectivity (VMHC) approach combined with resting-state functional magnetic resonance imaging to investigate changes in interhemispheric functional homotopy in 60 OCD patients and 60 healthy controls. Compared with the healthy controls, the OCD patients showed decreased VMHC values in the thalamus. The results suggest that changes in functional homotopy in the CSTC circuitry may be involved in the pathophysiologic mechanism of OCD. Our findings provide new evidence of abnormalities in the CSTC circuitry in OCD.
Keywords
Obsessive-compulsive disorder, Voxel-mirrored homotopic connectivity, Functional homotopy, Cortico-striato-thalamo-cortical circuitry, Resting-state
Introduction
Obsessive-compulsive disorder (OCD) is a debilitating disorder characterized by recurrent and persistent thoughts, images or urges (obsessions), and/or repetitive, time-consuming behaviors and rituals (compulsions) [1]. The prevalence rate of OCD is about 2%–3%, and about 50 million people worldwide suffer from the disease [2]. Patients with OCD find it difficult to control unwanted thoughts and behaviors, which cause great distress and can have serious effects on their work, study and interpersonal communication [1]. Therefore, there is increasing interest in understanding the neural mechanisms underlying OCD.
Although the mechanisms of OCD are still unclear, previous studies have used advanced neuroimaging approaches to reveal structural and functional brain changes in OCD patients. Decreased gray matter volumes in the orbitofrontal cortex, dorsolateral prefrontal cortex and anterior cingulate cortex, and increased gray matter volumes in the putamen, caudate and thalamus have been reported in previous OCD studies [3,4]. Local abnormal regional functional characteristics in the orbitofrontal cortex, anterior cingulate cortex, caudate and thalamus have been detected in OCD patients [5-7]. Moreover, changes in the resting-state functional connectivity (RSFC) among these brain regions have also been reported [8-11]. In sum, previous neuroimaging studies emphasize the abnormal structure and function of the cortico-striato-thalamo-cortical (CSTC) circuitry in OCD. It is also noteworthy that both increased and decreased fractional anisotropy values in the corpus callosum were reported in a diffusion tensor imaging study in OCD patients [12], which suggests that changes in the microstructure of the corpus callosum are involved in the process of obsessions and compulsions [13]. The corpus callosum, which is the largest area of white matter and is composed of association fibers in both hemispheres, plays an important role in interhemispheric communication and cognitive processes [14]. Altered white matter integrity in the corpus callosum may affect interhemispheric functional interactions [14-16], which are important for the integrity of brain functions [17]. However, OCD-related alterations in functional interactions between the cerebral hemispheres are rarely explored directly.
Resting-state functional magnetic resonance imaging (RS-fMRI) can reveal patterns of coherent spontaneous or intrinsic brain activities, and can be used to directly explore interhemispheric functional interactions. As one of the most essential properties of the brain’s intrinsic functional architecture, functional homology reveals highly correlated spontaneous activity between corresponding regions of the cerebral hemispheres, and can be evaluated using the RSFC approach [18-20]. A new measure, voxel-mirrored homotopic connectivity (VMHC), can quantify the homotopic patterns of RSFC by providing a voxelwise measure of connectivity between each voxel in one brain hemisphere and its mirrored voxel in the other [17]. Recently, the VMHC method has provided a variety of new disease-related findings on functional homology in cocaine addiction [15], schizophrenia [21], major depressive disorder [22,23], bipolar II disorder [24], autism [25] and drug-naive somatization disorder [26]. These findings demonstrate that VMHC can be regarded as a reliable and feasible measure to detect alterations in the functional homology between cerebral hemispheres in OCD patients.
Selective serotonin reuptake inhibitors (SSRI) are commonly used to treat OCD patients. Previous studies have shown that SSRI alter the structure and function of brain areas within the CSTC circuitry, such as decreased thalamic volume and local spontaneous activity in the dorsolateral prefrontal cortex and orbitofrontal cortex, and reduced RSFC between the orbitofrontal cortex and the striatum, and in the dorsomedial prefrontal cortex [27-31]. SSRI treatment also elevates the small-world efficiency, modular organization, and degree of connectivity in OCD patients [32]. However, whether SSRI change the functional homology between cerebral hemispheres in OCD patients in a resting-state remains unclear.
In the present study, we used RS-fMRI combined with the VMHC approach to explore changes in interhemispheric functional homotopy in OCD patients. We compared the VMHC differences between OCD patients and healthy controls (HCs), and between SSRI-treated and drugnaive OCD patients. The study aimed to discover changes in the spontaneous functional homology within the CSTC circuitry, and to examine whether SSRI treatment affects the altered functional architecture in OCD. Moreover, given the importance of interhemispheric interaction for cognitive processing, we expected to find a relationship between altered VMHC values and the clinical characteristics of OCD patients.
Material and Methods
▪ Subjects
Sixty-five OCD patients were recruited from the Qiqihar Mental Health Center and the Fourth Affiliated Hospital of Qiqihar Medical University. Data from five patients were excluded because of poor quality (see data acquisition and preprocessing). Of the remaining 60 patients, 20 had taken no medication for OCD and 40 were on stable doses of SSRI at the time of the scan. Sixty age- and gender-matched HCs from the local community were also recruited. All patients and HCs were right-handed, 18–60 years old and were diagnosed with the Structured Clinical Interview for DSM-IV Axis I Disorders Patient Edition and Non-patient Edition, respectively.
The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) [33], the 17-item Hamilton Rating Scale for Depression (HAMD) [34] and the Hamilton Anxiety Rating Scale (HAMA) [35] were used to assess the clinical characteristics of all subjects. Patients who scored ≥ 16 on the Y-BOCS scale and < 18 on the HAMD- 17 scale were included. None of the patients had a major physical disease, a history of neurological disorders or a history of psychiatric disorders other than OCD. The HCs had no neurological illness, major physical diseases, psychiatric disorders or family history of major psychiatric disorders. The study was approved by the Research Ethics Committee at Qiqihar Medical University. Written informed consent was obtained from all subjects.
▪ Data acquisition and preprocessing
The RS-fMRI images were acquired using a 3.0-Tesla GE 750 Signa-HDX scanner (General Electric Healthcare, Waukesha, Wisconsin) at the Third Affiliated Hospital of Qiqihar Medical University. An echo-planar imaging sequence was used for the RS-fMRI scans. The parameters were 33 axial slices, TR = 2000 ms, TE = 30 ms, FA = 90°, thickness/gap = 3.5/0.6 mm, FOV = 200 × 200 mm2, in-plane resolution = 64 × 64 and 240 volumes in total (8 min). All subjects were instructed to close their eyes and to lie as still as possible and relax but avoid falling asleep during scanning.
Data Processing & Analysis for (Resting-State) Brain Imaging (DPABI) [36] software was used for image preprocessing, including removal of the first 10 time points, slice timing, realigning, nuisance covariates regression (six head motion parameters, global mean time course, white matter time course and cerebrospinal fluid time course), normalized using the echo-planar image template and resampled to a voxel size of 3×3×3 mm3 and smoothed with a 4-mm full-width halfmaximum Gaussian kernel. Then, the signal was linearly detrended and band pass filtered at 0.01- 0.08 Hz to reduce low frequency drifts and high frequency physiological noise (i.e., respiratory and cardiac) [37]. The scrubbing procedure was conducted with a framewise displacement (FD) measure (with a threshold of 0.5 together with one preceding and two subsequent volumes) [38]. The FD of head position calculates the sum of the absolute values of the differential of the realignment estimates in the six parameters, which indicate the translational and rotational displacements of the head from a fixed position in space. Rotational displacements were calculated at a 50 mm radius [38]. Five OCD patients were excluded because more than 33% of the volumes were removed. We got the T1 images in Montreal Neurological Institute (MNI) space for each subjects, and created a mean T1 image template by averaging across all the subject. The mean T1 template was averaged with its flipped version to create a symmetric T1 template. Then, we normalized the T1 image in MNI space for each subject to the symmetric T1 template and applied the transformations to compute the homotopic connectivity [17].
▪ Voxel-mirrored homotopic connectivity
VMHC analysis was also performed using DPABI software [36]. Individual VMHC maps were computed as the Pearson correlation between a given voxel and a corresponding voxel in the mirrored hemisphere. The correlation values were transformed to standard z-values to achieve normality. The resultant values generated the VMHC maps and were used for within group and group-level (between OCD patients and HCs) comparisons. The results of one-sample t tests were used to identify the individual VMHC maps. Independent two-sample t tests were performed on the VMHC maps within a mask generated by selecting the voxels that showed significant VMHC in any of the two groups. Considering that SSRI may affect the restingstate spontaneous interhemispheric functional homotopy, two-sample t tests were used to compare differences in the VMHC results between SSRI-treated and drug-naïve OCD patients, between drug-naïve OCD patients and HCs, and between SSRI-treated OCD patients and HCs, respectively. Gaussian Random Field theory correction was used to assess statistical significance, with a voxel p value of < 0.001 and a cluster p value of < 0.05 (two tailed).
▪ Seed-based functional connectivity in the resting-state
Brain regions showing significant betweengroup VMHC difference were defined as seed ROIs. A reference time-course was obtained by averaging the time series of all voxels in each ROI. Pearson’s correlation analysis was conducted between each ROI reference timecourse and the time series of all other voxels in the whole brain in a voxel-wise manner. Six head motion parameters, the global mean, white matter and cerebrospinal fluid timecourse were regressed. The resulting r values were normalized to Z-values to facilitate the comparison. For each group, one-sample t tests were used to identify the individual z maps. Independent two-sample t tests were performed on the functional connectivity maps for each seed within a mask generated by selecting the voxels that showed significant positive or negative functional connectivity in any of the two groups (combined set of onesample t-test results). The significance level was set at p < 0.05 (Gaussian random field corrected, two-tailed).
▪ Correlations between VMHC and clinical symptoms in OCD patients
Partial correlation analysis was used to assess the relationship between clinical symptoms (YBOCS total, obsession and compulsion scores) and VMHC values that showed significant group differences. The total HAMA and HAMD scores and framewise-dependent values were considered as covariates to control for comorbid, nonspecific anxiety, depression symptoms and average motion. The threshold was set at p < 0.05/3 (0.017) (Bonferroni corrected).
Results
Demographic and clinical data
The subjects’ demographic and clinical data are displayed in Table 1. The patients and controls showed no significant differences in age, sex, years of education or framewise-dependent values (all p > 0.05).
| OCD (n = 60) | HCs (n = 60) | p | |
|---|---|---|---|
| Age (years) | 28.90 ± 8.46 | 28.24 ± 7.31 | 0.649 |
| Gender (male/female) | 44/16 | 44/16 | 1.000 |
| Education level (years) | 13.47 ± 2.69 | 14.07 ± 2.86 | 0.240 |
| Illness duration (months) | 79.55 ± 70.68 | ||
| Y-BOCS score | |||
| Total | 23.65 ± 7.91 | 1.00 ± 0.76 | < 0.001 |
| Obsessions | 13.58 ± 3.69 | 0.23 ± 0.50 | < 0.001 |
| Compulsions | 10.07 ± 6.11 | 0.77 ± 0.70 | < 0.001 |
| HAMD score | 9.88 ± 5.86 | 1.33 ± 1.02 | < 0.001 |
| HAMA score | 12.45 ± 7.82 | 1.18 ± 1.14 | < 0.001 |
| Mean FD | 0.12 ± 0.55 | 0.14 ± 0.74 | 0.082 |
Table 1: Demographic and clinical data of participants.
▪ Group differences in VMHC between OCD patients and HCs
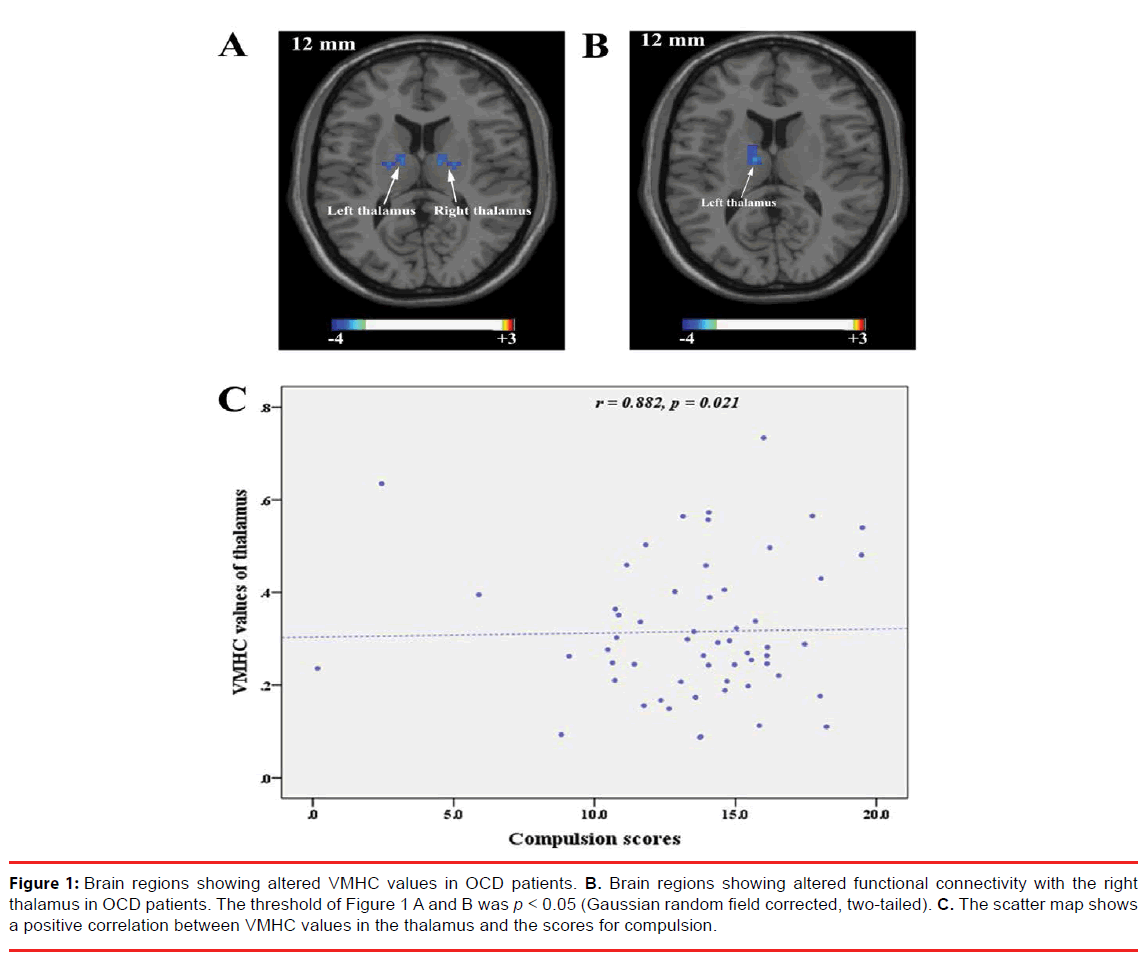
Figure 1A and Table 2 show the group comparisons of VMHC values between the OCD patients and HCs. The OCD patients exhibited significantly lower VMHC values in the thalamus than the HCs. No region showed greater VMHC values in the OCD group than in the HC group.
| Brain regions | Brodmann area | Cluster size (voxels) | MNI coordinates (x, y, z) |
t value |
|---|---|---|---|---|
| VMHC | ||||
| Thalamus | 26 | ±12, -6, 12 | -4.05 | |
| Positive functional connectivity in the resting-state with the right thalamus | ||||
| Thalamus | 33 | -12, -9, 12 | -4.35 | |
Table 2: Brain regions showing altered VMHC values and functional connectivity in OCD patients.
Figure 1: Brain regions showing altered VMHC values in OCD patients. B. Brain regions showing altered functional connectivity with the right thalamus in OCD patients. The threshold of Figure 1 A and B was p < 0.05 (Gaussian random field corrected, two-tailed). C. The scatter map shows a positive correlation between VMHC values in the thalamus and the scores for compulsion.
▪ Seed-based functional connectivity in the resting-state
As mentioned above, the thalamus exhibited lower VMHC values in the OCD group. We then explored the whole brain functional connectivity with the bilateral thalamus as the ROI. For the right thalamus, the left thalamus showed decreased positive functional connectivity in OCD patients (Table 2 and Figure 1B). For the left thalamus, no brain regions showed a difference in positive or negative functional connectivity in OCD patients.
▪ Group differences in VMHC and functional connectivity in SSRI-treated and drug-naïve OCD patients
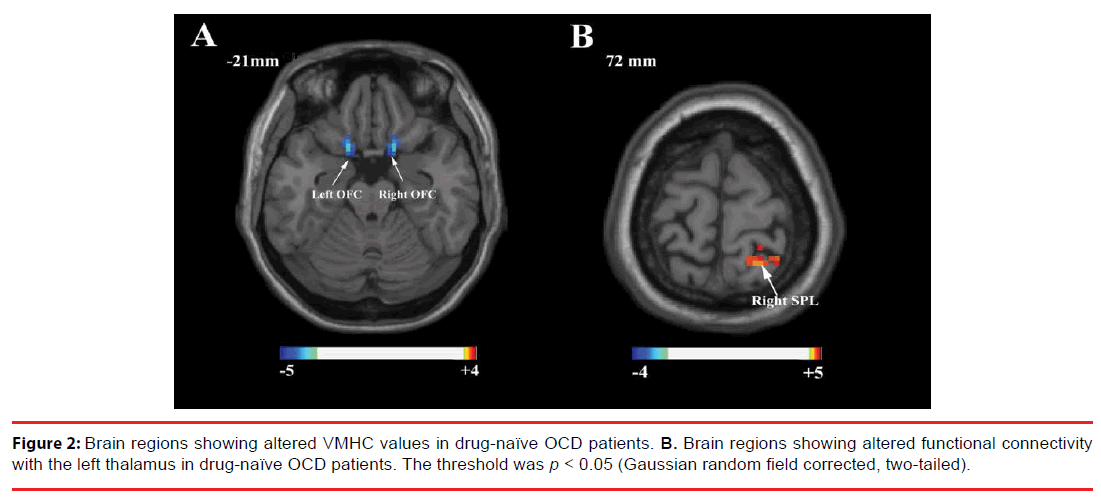
Drug-naïve OCD patients exhibited significantly lower VMHC values in the orbital frontal cortex and increased positive functional connectivity between the left thalamus and right superior parietal lobule than the HCs (Table 3 and Figure 2). SSRI-treated OCD patients showed no difference in VMHC and functional connectivity values compared with the HCs. No brain regions showed differences in VMHC and functional connectivity values between SSRItreated and drug-naive OCD patients.
| Brain regions | Brodmann area | Cluster size (voxels) | MNI coordinates (x, y, z) |
t value |
|---|---|---|---|---|
| VMHC | ||||
| Orbital frontal cortex | 11 | 22 | ±15, 15, -21 | -5.46 |
| Positive functional connectivity in the resting-state with the left thalamus | ||||
| Superior parietal lobule | 5 | 78 | 18, -51, 72 | 4.59 |
Table 3: Brain regions showing altered VMHC values and functional connectivity in drug-naïve OCD patients.
▪ Relationship between VMHC and clinical symptoms
The VMHC values in the thalamus were positively correlated with the scores for compulsion (r = 0.882, p = 0.021), but the correlation was no longer significant after Bonferroni correction (p > 0.017) (Figure 1C).
Discussion
In the current study, we investigated the alterations in interhemispheric functional homotopy in OCD patients using the VMHC approach with a relatively large sample. As hypothesized, we found that OCD patients showed decreased VMHC in the thalamus (a key node in the CSTC circuitry) compared with the HCs. Inconsistent with our hypotheses, no brain regions showed a difference in VMHC between the SSRI-treated and drug-naive patients, and we found no significant relationship between VMHC values and clinical symptoms in the OCD patients. Further analysis demonstrated that the right thalamus showed decreased positive functional connectivity with the left thalamus in OCD patients. To the best of our knowledge, this is the first study to use VMHC analysis to explore functional homotopy in OCD patients.
As an important part of the CSTC circuitry and a sensory and motor gateway, the thalamus provides specific channels from the basal ganglia to the cortical motor areas, and is responsible for sensory inputs and interactions between the cortex and basal ganglia, thus permitting the thalamus to mediate cognition and behavior [39,40]. The decreased interhemispheric VMHC values in the thalamus found in the present study indicate a reduced functional strength between the left and right sides of the thalamus. The decreased strength of homotopic RSFC in the thalamus may lead to dysfunction in the communication or integration of cognitive and behavioral information between the bilateral thalamus [17]. Furthermore, the inefficient gating at the thalamic level may result in hyperactivity within the CSTC circuitry in OCD [41], which could be involved in its pathophysiological mechanism. In the present study, although the positive correlation between decreased VMHC in the thalamus and compulsion disappeared after Bonferroni correction, we speculate that the impaired intrinsic interhemispheric functional homotopy in the thalamus might be associated with the regulation of compulsive behavior. Previous studies have also demonstrated increased gray matter volume, decreased regional spontaneous neuronal activity and homogeneity, and reduced glutamate level in the thalamus, which were also related to the clinical symptoms of OCD [3,6,7,12,42]. Taking these findings together, we have reason to speculate that the anatomical and functional abnormalities in the thalamus are widely implicated in OCD, and our findings provide new evidence that abnormalities in the CSTC circuitry underlie the pathophysiological mechanisms of OCD.
To explore the effect of SSRI on the intrinsic functional homology between cerebral hemispheres, we compared the VMHC values between with SSRI-treated and drug-naive OCD patients and found no differences in VMHC in any brain region. Previous studies have demonstrated that SSRI treatment affects the thalamic volume [30,31], but our results suggest that it may have no effect on the interhemispheric functional homology of OCD. However, we only compared the intrinsic functional homology between SSRI-treated and drug-naive OCD patients, rather than comparing the same group of patients before and after SSRI treatment. In present study, it’s interesting that drug-naïve OCD patients exhibited significantly lower VMHC values in the orbital frontal cortex and increased positive functional connectivity between the left thalamus and right superior parietal lobule, however, SSRI-treated OCD patients showed no any difference in VMHC values and functional connectivity compared with the HCs. It is cautious to infer that SSRI may affect the reduced functional homology in the CSTC circuitry in OCD patients because the sample is inconsistent. The effect of SSRI on the intrinsic interhemispheric functional homotopy in OCD patients should be further explored in longitudinal studies with the same OCD sample.
We also found that the right thalamus showed decreased positive functional connectivity with the left thalamus in the OCD patients. Functional connectivity refers to the temporally correlated activity between remote brain regions, and positive functional connectivity may integrate neuronal activities for similar goals [43]. Decreased positive functional connectivity may suggest that the cooperative and integrative relationship between the left and right thalamus may be destroyed, which may diminish the filtration and suppression of irrelevant information in the thalamus [44], resulting in the excessive, unnecessary thoughts and behaviors shown by OCD patients. The decreased positive functional connectivity between the right and left thalamus may further support the decreased intrinsic interhemispheric functional homotopy in the thalamus in OCD patients. However, there were no brain regions showing altered resting-state functional connectivity with the left thalamus as seed in the OCD patients. Previous researches found that the left and right brain regions with changed VMHC values showed different resting-state functional connectivity pattern [22], and the left and right thalamus may have diverse whole-brain functional connectivity patterns in Alzheimer’s disease [45]. Based on the present research results, we infer that the left and right thalamus with changed VMHC values may also have different functional connectivity pattern at resting-state in OCD patients.
The present study was strengthened by the relatively large sample size. However, some limitations should be taken into consideration. First, neuropsychological data, especially cognitive and behavioral information, were not collected in our study. The relationship between deficits in functional homotopy and cognitive dysfunction should be investigated in future research. Second, white matter diffusion and gray matter volume were not analyzed in the present study, which makes it impossible for us to know the relationship between VMHC and white matter diffusion or gray matter volume. Therefore, multimodal MRI technology involving voxel-based morphometry, diffusion tension imaging, and RS-fMRI is needed to integrate structural and functional interhemispheric connectivity and thus explore the pathophysiology of OCD in greater depth.
Conclusions
In summary, the present study revealed decreased VMHC values in the thalamus in OCD patients, suggesting that changes in the functional homotopy in the CSTC circuitry might be involved in the pathophysiological mechanism of OCD. Our findings provide new evidence of abnormalities in the CSTC circuitry in OCD, which should improve the understanding of OCD.
Acknowledgments
This work was supported by Heilongjiang Natural Science Foundation (H2016100). We thank for all the research participants who willingly gave their time to provide data. We thank Rachel Baron, PhD from Liwen Bianji, Edanz Editing China (www. liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Association Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). APA, Washington (2013).
- Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol.Psychiatry 15(1), 53-63 (2010).
- Rotge JY, Guehl D, Dilharreguy B, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol. Psychiatry 65(1),75-83 (2009).
- Peng Z, Lui SS, Cheung EF, et al. Brain structural abnormalities in obsessive-compulsive disorder: converging evidence from white matter and grey matter. Asian J Psychiatr 5(4), 290-296 (2012).
- Ping L, Su-Fang L, Hai-Ying H, et al. Abnormal Spontaneous Neural Activity in Obsessive-Compulsive Disorder: A Resting-State Functional Magnetic Resonance Imaging Study. PLoS. ONE 8(6), e67262 (2013).
- Niu Q, Yang L, Song X, et al. Abnormal resting-state brain activities in patients with first-episode obsessive-compulsive disorder. Neuropsychiatr Dis Treat 13, 507-513 (2017).
- Qiu L, Fu X, Shuai W, et al. Abnormal regional spontaneous neuronal activity associated with symptom severity in treatment-naive patients with obsessive-compulsive disorder revealed by resting-state functional MRI. Neurosci. Lett 640, 99-104 (2017).
- Posner J, Marsh R, Maia TV, et al. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp 35(6), 2852-2860 (2014).
- Chen Y, Juhas M, Greenshaw AJ, et al. Abnormal resting-state functional connectivity of the left caudate nucleus in obsessive-compulsive disorder. Neurosci. Lett 623, 57-62 (2016).
- Sakai Y, Narumoto J, Nishida S, et al. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur. Psychiatry 26 (7), 463-469 (2011).
- Harrison BJ, Soriano-Mas C, Pujol J, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch. Gen. Psychiatry 66 (11), 1189-1200 (2009).
- Eng GK, Sim K, Chen SH. Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: an integrative review. Neurosci Biobehav Rev 52 (2), 233-257 (2015).
- Jose D, Narayanaswamy JC, Agarwal SM, et al. Corpus callosum abnormalities in medication-naive adult patients with obsessive compulsive disorder. Psychiatry Res 231 (3), 341-345 (2015).
- Gazzaniga MS. Cerebral specialization and interhemispheric communicationDoes the corpus callosum enable the human condition? Brain 123 (7), 1293-1326 (2000).
- Kelly C, Zuo XN, Gotimer K, et al. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol. Psychiatry 69 (7), 684-692 (2011).
- Yu D, Yuan K, Bi Y, et al. Altered interhemispheric resting-state functional connectivity in young male smokers. Addict Biol 5 (2017).
- Zuo XN, Kelly C, Di MA, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 30 (45), 15034-15043 (2010).
- Salvador R, Suckling J, Schwarzbauer C, et al. Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos Trans. R. Soc. Lond., B, Biol. Sci. 360 (1457), 937-946 (2005).
- Marshall O, Uh J, Lurie D, et al. The influence of mild carbon dioxide on brain functional homotopy using resting‐state fMRI. Hum Brain Mapp 36 (10), 3912-3921 (2015).
- Stark DE, Margulies DS, Shehzad ZE, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J. Neurosci. 28 (51), 13754-13764 (2008).
- Guo W, Xiao C, Liu G, et al. Decreased resting-state interhemispheric coordination in first-episode, drug-naive paranoid schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 48 (1433), 14-19 (2014).
- Guo W, Liu F, Dai Y, et al. Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 41 (4), 24-29 (2013).
- Guo W, Feng L, Xue Z, et al. Decreased Interhemispheric Coordination in Treatment-Resistant Depression: A Resting-State fMRI Study. PLoS. ONE 8(8), e71368 (2013).
- Wang Y, Zhong S, Jia Y, et al. Reduced interhemispheric resting-state functional connectivity in unmedicated bipolar II disorder. Acta Psychiatr Scand 132 (5), 400-407 (2015).
- Anderson JS, Druzgal TJ, Froehlich A, et al. Decreased interhemispheric functional connectivity in autism. Cereb. Cortex 21(5), 1134-1146 (2011).
- Su Q, Yao D, Jiang M, et al. Decreased interhemispheric functional connectivity in insula and angular gyrus/supramarginal gyrus: Significant findings in first-episode, drug-naive somatization disorder. Psychiatry Res 248, 48-54 (2016).
- McCabe C, Mishor Z, Filippini N,et al. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol. Psychiatry 16 (6), 592-594 (2011).
- McCabe C, Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage 57 (4), 1317-1323 (2011).
- Kennedy SH, Konarski JZ, Segal ZV, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry 164 (5), 778-788 (2007).
- Psychiatry AOG. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Arch. Gen. Psychiatry 57 (5), 449-456 (2000).
- Atmaca M, Mermi O, Yildirim H, Gurok MG. Orbito-frontal cortex and thalamus volumes in obsessive-compulsive disorder before and after pharmacotherapy. Brain Imaging Behav 10 (3), 669-674 (2016).
- Shin DJ, Jung WH, He Y, et al. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol. Psychiatry 75 (8), 606-614 (2014).
- Zhang Y, Meng F, Cui Y, et al. A study on the clinical reliability and validity of modified Yale Brown Obsessive Compulsive Scale. Chinese Journal of mental health, 10 (5), 205-207 (1996).
- Tang L, Zhang M. Hamilton Depression Scale. Shanghai archives of psychiatry (2), 61-64 (1984).
- Tang L, Zhang M. Hamilton Anxiety Scale. Shanghai archives of psychiatry (2), 64-65 (1984).
- Yan CG, Wang XD, Zuo XN, et al. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 14 (3), 339-351(2016).
- Biswal B, Yetkin FZ, Haughton VM, et al.. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34 (4), 537-541 (1995).
- Power JD, Barnes KA, Snyder AZ, et al.. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59 (3), 2142-2154 (2012).
- Lacerda ALT, Dalgalarrondo P, Caetano D, et al. Elevated thalamic and prefrontal regional cerebral blood flow in obsessive–compulsive disorder: a SPECT study. Psychiatry Res 123(2), 125-134 (2003).
- Rossi S, Bartalini S, Ulivelli M, et al. Hypofunctioning of Sensory Gating Mechanisms in Patients with Obsessive-Compulsive Disorder. Biol. Psychiatry 57(1), 16-20 (2005).
- Del Casale A, Kotzalidis GD, Rapinesi C, et al. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology 64 (2), 61-85 (2011).
- Zhu Y, Fan Q, Han X, et al. Decreased thalamic glutamate level in unmedicated adult obsessive–compulsive disorder patients detected by proton magnetic resonance spectroscopy. J Affect Disord 178 (1), 193-200 (2015).
- Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A 102 (27), 9673-9678 (2005).
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst 18 (8), 386-404 (2002).
- Zhou B, Liu Y, Zhang Z, et al. Impaired functional connectivity of the thalamus in Alzheimer's disease and mild cognitive impairment: a resting-state fMRI study. Curr Alzheimer Res 10 (7), 754-766 (2013).