Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Pregabalin, Duloxetine, and Diazepam Selectively Modulate Acid-Induced Hyperalgesia and Anxio-Depressive Comorbidity in Rats
- Corresponding Author:
- Fu-Zen Shaw, PhD
Department of Psychology, National Cheng Kung University
No 1 University Road, Tainan 70101, Taiwan
Tel: +886-62004555
Fax: +886-62752029
Abstract
Abstract
Objective: Patients with chronic widespread pain (CWP) syndrome often have comorbid with depression/ anxiety symptoms and unsatisfied. Effects of pregabalin, duloxetine and diazepam in the well-established acid-induced CWP model remain unclear. This study aimed to assess these medicines on mechanical hyperalgesia and psychiatric comorbidity in this CWP model.
Methods: The CWP model was elicited by repeated injections of acidic solution into unilateral gastrocnemius muscle of a rat. Each medicine was administered orally via gavage twice per day for >2 weeks. Paw withdrawal threshold (PWT) was evaluated by von Frey filaments. Anxiety-like behavior was assessed by an elevated plus maze. Despair mood and anhedonia of depression-like behavior were evaluated using the forced swimming and sucrose preference tests, respectively.
Results: Rats receiving acid injections displayed bilateral hyperalgesia and anxio-depressive comorbidity. Pregabalin significantly increased PWTs and ameliorated anxiety-like and anhedonic behaviours. Duloxetine significantly increased PWTs and ameliorated depressionlike but not anxiety-like behaviour. Diazepam did not alter PWTs but remedied anxiety-like and anhedonic behaviours. Pregabalin, duloxetine, and diazepam revealed different effects on hyperalgesia and anxio-depressive comorbidity in the acid-induced CWP model.
Conclusion: Our results strengthen the acid-induced pain model as humans with CWP on additional evidences of face and predictive validities. This study provides information of these medicines on selective treatment of pain and psychiatric comorbidity.
Keywords
Muscle pain, Fibromyalgia, Pregabalin, Duloxetine, Diazepam, Anxiety, Depression
Introduction
Chronic widespread pain (CWP) syndromes, such as fibromyalgia (FM) and myofacial pain syndrome, are characterized by widespread and long-lasting musculoskeletal pain. FM affects 2% of the general population and a considerable proportion of patients present with symptoms of mood disorders, particularly anxiety and depression [1,2]. Thus, CWP syndromes are associated with significant disability, self-injury and medical costs [3]. Several medicines, such as duloxetine and pregabalin, are used in patients with CWP syndromes [4]. Both pregabalin and duloxetine fibromyalgia clinical trials reveal that the pain relief is not related to alleviation of anxiety or depression symptom [5]. Recently, a clinical study indicates that combination of pregabalin with duloxetine for fibromyalgia increases multiple clinical outcomes [6]. However, there is little guideline on these medicines to manage pain and psychiatric comorbidity in fibromyalgia patients because of divergent comorbid symptoms (e.g. psychiatric disorder, sleep disturbance, etc.) [7,8]. Animal models with psychiatric comorbidity play an important role in elucidating the psychophysiology of CWP syndromes in terms of various medicines [9].
An animal model with CWP syndromes has been developed using repeated acid injections to the gastrocnemius muscle in two- to five-day intervals to produce a long-lasting, bilateral, mechanical and muscular hyperalgesia but not thermal hyperalgesia, without motor deficits or tissue damage [10,11]. Recently, acid-induced CWP animal model has shown comorbid anxiety- and depression-like behaviors [12]. This CWP animal model has been used for studying the mechanisms of peripheral and central sensitization [13-15]. Previous studies have shown acute effect of pregabalin on reducing hyperalgesia in the acid-induced CWP model [11]. Acute administration of diazepam has shown little effect on acid-induced hyperalgesia [16]. In contrast to acute treatment of these medicines on hyperalgesia, there is however not systematically investigated about the effects of chronic administration of pregabalin, duloxetine and diazepam on both hyperalgesia and psychiatric comorbidity in the CWP animals.
In the current study, effects of pregabalin, duloxetine and diazepam were investigated on both acid-induced hyperalgesia and anxio-depressive behaviors in the CWP animal model through a chronic oral administration via gavage. We hypothesized that these drugs would selectively modulate hyperalgesia and comorbidity of anxiety- and depression-like behaviors in the acid-induced CWP animal model. The results of this study provided predictive validity between acid-induced muscle pain in CWP animal model and patients with CWP syndromes and also extended our understanding of these medicines on CWP syndromes and psychiatric comorbidity.
Methods
Male Sprague-Dawley rats (8-9 weeks old) were kept in a sound-attenuated room under a 12/12- h light/dark cycle (lights on at 06:00-18:00) with food and water provided ad libitum. The rats were randomly assigned into a group receiving the vehicle (pH 7.2) or acidic saline (pH 4.0) at a ratio of 1:1.1 because of unsuccessful pain induction in previous studies [10,16]. The Institutional Animal Care and Use Committee reviewed and approved the experimental procedure. All experiments complied with the guidelines for the ethical use of animals of the US National Institutes of Health. The entire experimental design was conducted according to institutional guidelines and followed the 3R’s principles (i.e., replacement, refinement, and reduction) for animal welfare. According to our previous study [12], ten rats with successful pain induction in each group were used.
▪ Induction of muscle pain
The method used to induce muscle-mediated chronic pain was as previously described in detail [10]. All rats were briefly anesthetized with vaporized isoflurane (3-5%). After the skin covering was shaved, left gastrocnemius muscle was injected with 100 μl neutral saline (pH 7.2) in the control group or 100 μl acidic saline in the experimental group, on days 1 and 6. Normal saline was adjusted with an 2-[N-morpholino] ethanesulfonic acid to pH 4.0±0.1 as acidic saline.
▪ Von Frey filament testing
Rats were placed in a Lucite cubicle on an elevated metal grid allowing for the stimulation on plantar surface of the paw. The grid hole diameter was 3 mm and the center distance between two consecutive holes was 5 mm. Rats were placed on the platform for adaptation to the apparatus for 5-10 min prior to the experimental measurement. Discrete von Frey filaments of varying bending forces (2, 4, 6, 8, 10, 15, and 26 g) were applied to plantar surface of the paw to assess the withdrawal response. A “response” to the stimuli was defined as an abrupt lifting of the foot upon application of the von Frey filament. A trial contained 5 von Frey stimuli, with an inter-stimulus interval of 5-6 minutes to reduce possible habituation. Von Frey testing forces were performed in an ascending sequence. Paw withdrawal threshold (PWT) was defined as the lowest force that elicited ≥3 withdrawals in 5 consecutive stimuli.
Von Frey nylon hairs were calibrated both prior to and throughout the time course of the study to ensure that consistent bending forces were routinely applied. The withdrawal threshold of the ipsilateral left hind paw was measured first, and then the contralateral right hind paw. Von Frey tests were employed at the times of D1, D2, D6, D7, D13, D14, D20, D21, D27, D28, D34, and D35.
▪ Body weight assessment
Body weight was assessed using a commercial scale at particular time points similar to those described for von Frey filament testing (Figure 1).
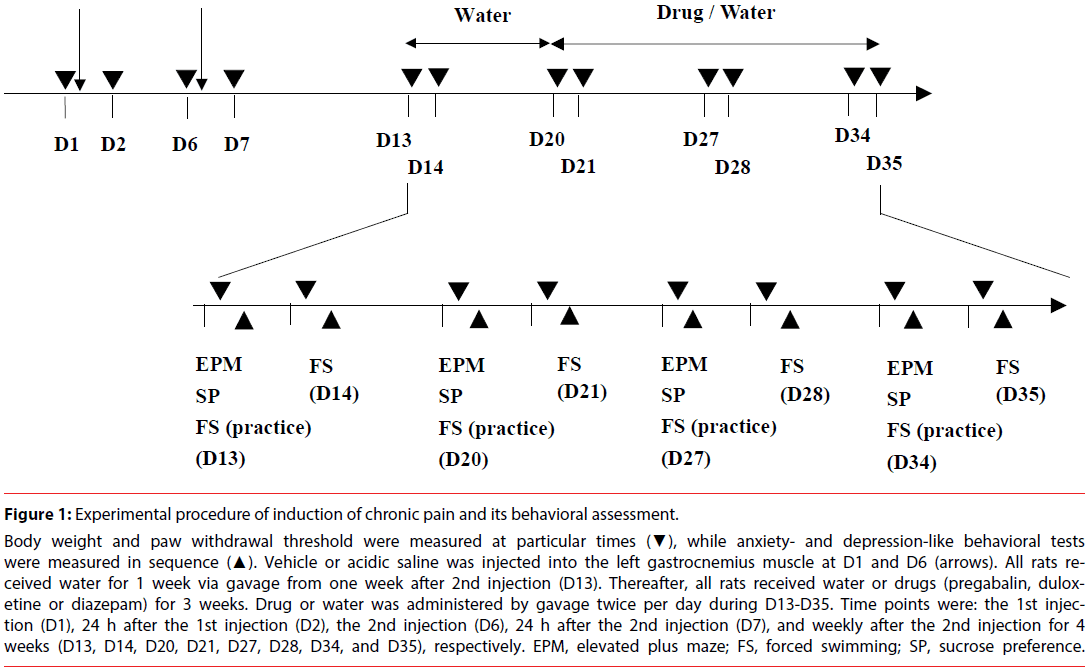
Figure 1: Experimental procedure of induction of chronic pain and its behavioral assessment.
Body weight and paw withdrawal threshold were measured at particular times (▼), while anxiety- and depression-like behavioral tests were measured in sequence (▲). Vehicle or acidic saline was injected into the left gastrocnemius muscle at D1 and D6 (arrows). All rats received water for 1 week via gavage from one week after 2nd injection (D13). Thereafter, all rats received water or drugs (pregabalin, duloxetine or diazepam) for 3 weeks. Drug or water was administered by gavage twice per day during D13-D35. Time points were: the 1st injection (D1), 24 h after the 1st injection (D2), the 2nd injection (D6), 24 h after the 2nd injection (D7), and weekly after the 2nd injection for 4 weeks (D13, D14, D20, D21, D27, D28, D34, and D35), respectively. EPM, elevated plus maze; FS, forced swimming; SP, sucrose preference.
▪ Behavioral tests
Three behavioral tests were used to evaluate anxiety- and depression-like behaviours of rats [12,17]: elevated plus maze test, forced swimming test, and sucrose preference test. The elevated plus maze test was used to measure anxious status for animals facing open environmental stimulus. The forced swimming test was used to assess the duration of immobility, which was analogous to a despair-like status. The sucrose preference test was a measure of the ‘hedonic’ state of an animal or the ability to experience pleasure. A decreased sensitivity to reward (i.e., anhedonia) is a fundamental feature of clinical depression. All behavior measures were taped, digitized, and analyzed using video tracking record system (TM-01, Singa, Taiwan).
▪ Elevated plus maze test
The elevated plus maze consisted of black polypropylene plastic which was elevated 68 cm above the floor. Each maze arm extended 45 cm from the junction area, which measured 13×13 cm. The open and closed arms were 13 cm wide, and the closed maze arms had walls extending 25 cm from the junction area. During testing, each rat was placed in the central square facing an open arm and was allowed 5 minutes to freely explore the maze. The alleys of the maze were thoroughly cleaned with an ethanol solution (60% volume) after the removal of each rat. Time spent on different sections of the maze (including the central area) and the numbers of open and closed arm entries were measured. The percentage of entries in open arms was calculated according to the following formula: percentage of open arm entry = (number of entries into the open arms/number of open+closed arm entries) × 100; percentage of time spent in open, centre, and closed sections of the maze = (location × 100/300). The percentage of venturing into open arms is validated for anxiety measure [18].
▪ Sucrose preference test
In the sucrose preference test, rats were placed in an unfamiliar test cage supplied with two identical bottles, one contained drinking water and the other sucrose solution (20%). All rats were allowed freely to approach the bottles for a period of 1 h after 23 h of food and water deprivation. The results were expressed as a percentage of sucrose preference according to the following formula: preference (%) for sucrose over water (calculated as [sucrose intake/ total fluid intake] × 100%). The preference for sucrose solution is a reliable hedonic index in rats [12,19].
▪ Forced swimming test
The forced swimming test apparatus was a plastic cylinder (47 cm height, 38 cm inside diameter) containing 38 cm of water at 25±1°C. The forced swimming test consisted of two phases. In the initial 15-min habituation session of the first day, which was excluded from the data analysis, the rats were individually forced to swim in a plastic cylinder. After a period of vigorous swimming, all of the rats reduced their movements to only those necessary to maintain their head above the water level, with no other displacement. The 5-min test sessions began 24 h later. The duration of immobilization was measured. After the test, rats were removed and dried with a towel before being returned to their home cages. Increased immobility in the forced swimming test was indicative of depression-like behavior [20].
▪ Drug administration
A chronic oral administration was used via gavage. Dosage used in rats based on the following equation: human dosage × human Km/ animal Km, where Km= weight/body surface area. In general, dosage of the rat was 6 folds as used in human [21]. Pregabalin (LYRICA 75 mg capsule; Pfizer), duloxetine (Cymbalta 30 mg capsule; Eli Lilly), and diazepam (Diapin 2 mg tablet; Souriree) were administered orally by gavage in a dose of 15 mg/kg [11], 3 mg/ kg [22], and 0.5 mg/kg [16], respectively. A single dose was administered throughout the experiment. All medicines mixed with 2 ml water were administered twice daily via gavage for >2 weeks in the acid group. The vehicle group received the same amount of water twice daily via gavage. The interval between the two drug/ water administrations in any given day was 4 h.
▪ Experimental procedure
All rats were placed in the recording room 1 week prior to the experiment for adaptation. Rats were placed in a Lucite cubicle on an elevated metal grid allowing stimulation of plantar surface of the hind paw for at least 20 min to allow them to become acclimatized to the test environment. This was done for 3 days before von Frey filament testing. Two intramuscular injections of pH 7.2 or pH 4.0 saline were employed at D1 and D6. Body weight and paw withdrawal threshold were measured at particular time points (Figure 1). All behavioral evaluations were performed at 14:00-17:00 to minimize circadian influences.
The study was designed explore long-term effect of drugs on hyperalgesia and anxio-depressive comorbidities. To this aim, anxio-depressive behavioral tests were repeatedly employed as previous studies [22-24]. The elevated plus maze, sucrose preference, and forced swimming tests were carried out 1 week after 2nd intramuscular injection in sequence. All rats received water twice daily per orally for 1 week. Subsequently, rats received water or drug via gavage from D20 for >2 weeks. Three behavioral tests were carried out once per week. A detailed flowchart of the entire experimental procedure is shown in Figure 1. Measures were performed before the 1st injection (D1), 24 h after the 1st injection (D2), before the 2nd injection (D6), 24 h after the 2nd injection (D7), and weekly after the 2nd injection for 4 weeks (D13, D14, D20, D21, D27, D28, D34 and D35), respectively.
Statistical analysis
Changes in body weight and indexes of the elevated plus maze test, the sucrose preference test, and forced swimming test in all groups were analyzed using two-way repeated measures analysis of variance (ANOVA) with one factor repetition, if appropriate, followed by post hoc Fisher’s LSD test. Temporal changes of the paw withdrawal thresholds in all groups were assessed by Friedman repeated measures ANOVA on rank, if appropriate, followed by Dunnett’s test. The paw withdrawal threshold among groups at a particular time point was analyzed by oneway ANOVA on rank (H-test) followed by post hoc Dunnett’s test. To further assess the stability of a within-subject experimental design, all indexes of the vehicle group throughout the recording period were analyzed by one-way repeated measure ANOVA. Data were expressed as the mean ± standard error of the mean (SEM). All statistics were carried out using Sigmaplot statistics. Statistical significance was set at p<0.05.
Results
In the present study, 56 rats (vehicle n=10, acid n=46) were used. Six rats receiving intramuscular injections of acidic saline failed to exhibit mechanical hyperalgesia. A hyperalgesia induction rate was 87% in the acid group. Thus, 40 rats with hyperalgesia were randomly assigned into the acid groups treated with water (n=10), pregabalin (n=10), duloxetine (n=10), or diazepam (n=10), respectively.
▪ Paw withdrawal threshold
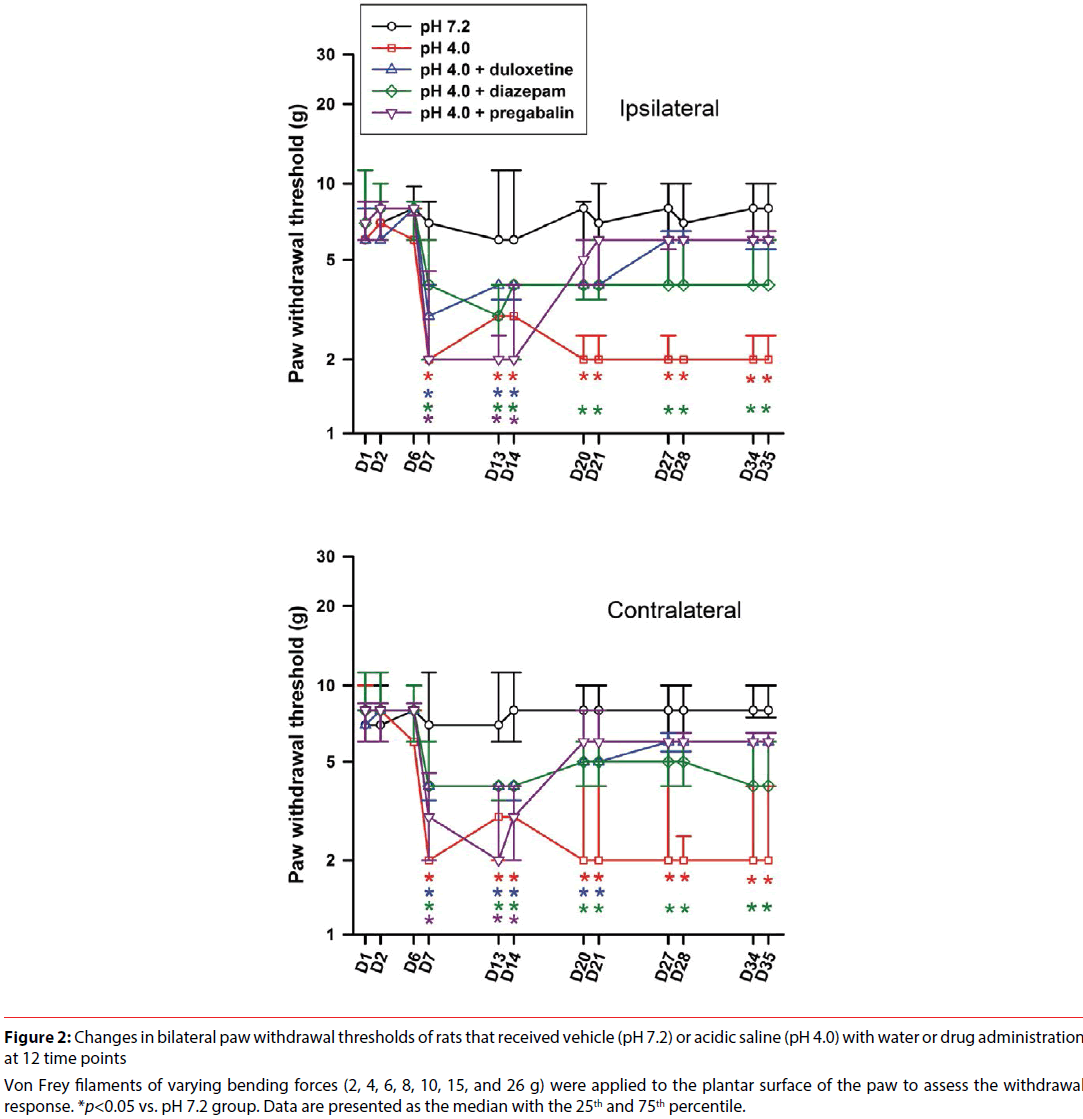
The withdrawal thresholds of bilateral hind paws of rats in all groups are shown in Figure 2. Paw withdrawal threshold of the vehicle group was consistent in bilateral hind limbs, and the threshold was primarily at 8 g throughout the measured period. There was no significant difference throughout the recording period in the vehicle group (ipsilateral, χ2(11)=6.261, p=0.855; contralateral, χ2(11)=9.432, p=0.582). In contrast, the paw withdrawal threshold of the acid group was at the same level as that of the vehicle group before the 2nd injection of acid saline. The paw withdrawal thresholds of bilateral hind limbs were reduced to 2-4 g after the 2nd injection of acid saline. This hyperalgesic response lasted for 4 weeks. Intramuscular administration of acidic saline produced longlasting and significant decreases in the paw withdrawal threshold (ipsilateral, χ2(11)=86.637, p<0.001; contralateral, χ2(11)=87.174, p<0.001). Paw withdrawal thresholds from D7 to D35 were significantly lower than its baseline (D1) in the acid group treated with water. In addition, significant differences between the vehicle and acid groups were also found at the same time points.
Figure 2: Changes in bilateral paw withdrawal thresholds of rats that received vehicle (pH 7.2) or acidic saline (pH 4.0) with water or drug administration at 12 time points
Von Frey filaments of varying bending forces (2, 4, 6, 8, 10, 15, and 26 g) were applied to the plantar surface of the paw to assess the withdrawal response. *p<0.05 vs. pH 7.2 group. Data are presented as the median with the 25th and 75th percentile.
Paw withdrawal threshold of the acid group treated with pregabalin revealed significantly temporal change (ipsilateral, χ2(11)=76.508, p<0.001; contralateral, χ2(11)=83.001, p<0.001). Compared with the vehicle group, the acid group treated with pregabalin had significantly lower paw withdrawal thresholds at D7, D13, and D14 prior to drug administration, and then showed a rapid reverse on bilateral withdrawal thresholds from D20, when rats started to receive pregabalin. Thus, pregabalin exhibited analgesic effect.
Paw withdrawal threshold of the acid group treated with duloxetine showed significantly temporal change (ipsilateral, χ2(11)=70.067, p<0.001; contralateral, χ2(11)=71.023, p<0.001). Compared with the vehicle group, the acid group treated with duloxetine had significantly lower paw withdrawal thresholds at D7, D13, and D14 prior to duloxetine administration. The acid group treated with duloxetine showed a progressive return pattern on the withdrawal thresholds D20 and D21 starting drug administration (significant difference existed in contralateral hind limb but not in ipsilateral hind limb) followed by no difference on paw withdrawal thresholds of bilateral hind limbs compared with those of the vehicle group. Thus, duloxetine showed analgesic response in this model.
Paw withdrawal threshold of the acid group treated with diazepam showed significantly temporal change (ipsilateral, χ2(11)=70.131, p<0.001; contralateral, χ2(11)=71.182, p<0.001). The acid group treated with diazepam showed significantly lower paw withdrawal thresholds compared with the vehicle group from D7-D35. The results indicated that diazepam had no effect on acid-induced hyperalgesia.
▪ Body weight
Body weights of the five groups progressively increased from 300 g to 370 g throughout the recording period (Figure S1). There were significantly difference in the factors of time (F(11,495)=283.189, p<0.001) and treatment×time (F(44,495) =1.426, p=0.041). Body weight had insignificant difference in the group factor (F(4,495)=2.113, p=0.095).
▪ Elevated plus maze test
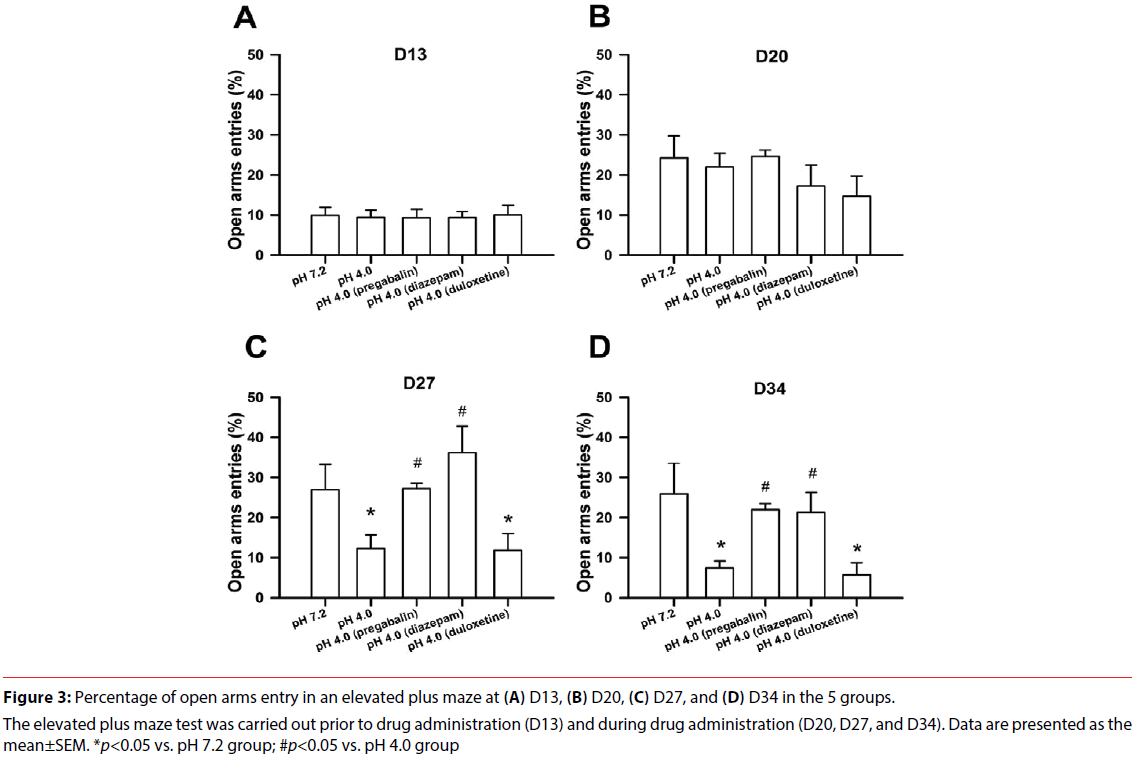
The percentages of open arms entry of an elevated plus maze at all measuring time points in all groups are shown in Figure 3. Total movement during the elevated plus maze test was not significantly different in the five groups. The percentage of open arms entry in the open arms was significant difference in the factors of treatment (F(4,135)=6.475, p<0.001), time (F(3,135)=10.344, p<0.001), and treatment×time (F(12,135)=2.186, p=0.016). There was no significant difference in all groups at D13 prior to drug administration, which indicated a well match at baseline among all groups. There was no significant difference on the percentage of open arms entry throughout the recording period in the vehicle group (F(3,27)=2.236, p=0.107), which indicated no alteration in repeated measures of an elevated plus maze. The percentage of open arms entry was significant difference throughout the recording in the acid group (F(3,27)=9.591, p<0.001). The percentage of open arms entry was not significantly different at D13 prior to drug administration. The acid group receiving water showed significantly lower percentage of open arms entry in the open arms at D27 and D34 compared with the vehicle group. The acid group exhibited anxiety-like behavior.
Figure 3: Percentage of open arms entry in an elevated plus maze at (A) D13, (B) D20, (C) D27, and (D) D34 in the 5 groups.
The elevated plus maze test was carried out prior to drug administration (D13) and during drug administration (D20, D27, and D34). Data are presented as the mean±SEM. *p<0.05 vs. pH 7.2 group; #p<0.05 vs. pH 4.0 group
The acid groups treated with pregabalin (F(3,27)=18.408, p<0.001) or with diazepam (F(3,27)=5.041, p=0.007) exhibited significant difference throughout the recording. The acid group receiving pregabalin or diazepam showed significantly higher percentage of open arms entry at D27 and D34 compared with the acid group receiving water. There was no difference between the vehicle group and the acid group treated with pregabalin or diazepam at D27 and D34. These data indicated that pregabalin or diazepam ameliorated anxiety-like behavior of the acid-induced CWP rats.
There was no significant difference on the percentage of open arms entry in the acid group treated with duloxetine (F(3,27)=0.850, p=0.479). The acid group receiving duloxetine showed significantly lower percentage of open arms entry at D27 and D34 compared with the vehicle group, which indicated anxiety-like behavior in the group receiving duloxetine.
In addition to the percentage of open arms entry in an elevated plus maze, other parameters are summarized in Table S1 (see supplementary results).
▪ Sucrose preference test
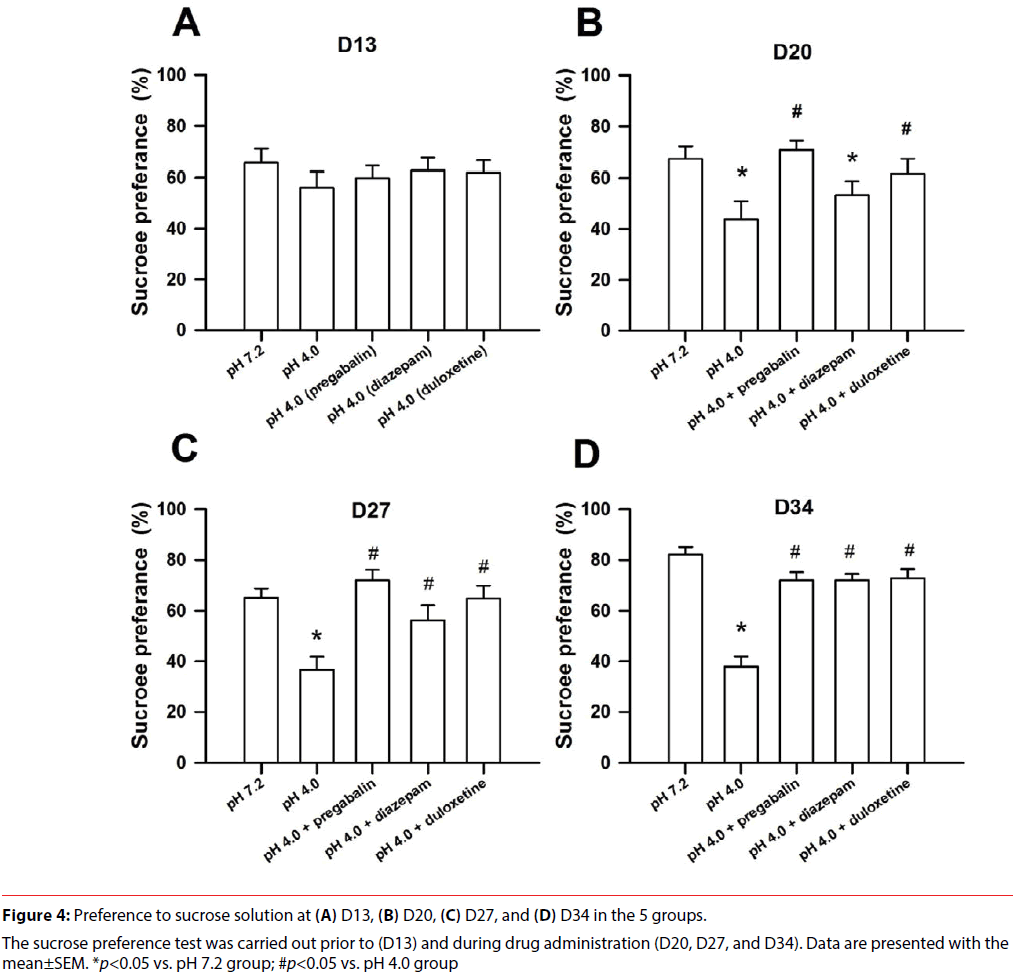
The results of the sucrose preference test at 4 time points in all groups are shown in Figure 4. Total liquid intake (including sucrose and water) in all groups fell in the range of 13.5- 18 ml during the sucrose preference test, and there was no difference in all groups (F(4,135)=2.271, p=0.076). Sucrose preference had significant differences in the factors of treatment (F(4,135)=16.199, p<0.001), time (F(3,135)=3.485, p=0.018), and treatment×time (F(12,135)=2.328, p=0.01). There was no significant difference in all groups at D13 prior to drug administration, which indicated a well match at baseline among all groups. There was significantly progressive increase in the sucrose preference throughout the recording period in the vehicle group (F(3,27)=3.834, p=0.021), which implied a hedonic preference. The acid group receiving water exhibited significant decrease in the sucrose preference throughout the recording (F(3,27)=3.163, p=0.041), which sluggested an anhedonic pattern. The acid group receiving water exhibited significantly lower sucrose preference at D20, D27, and D34 compared with the vehicle group and had no difference with 50% chance.
The acid group receiving duloxetine exhibited no significant difference throughout the recording (F(3,27)=1.175, p=0.338). The acid group receiving duloxetine had no difference at all timestamps compared with the vehicle group, but it exhibited significantly higher sucrose preference at D20, D27, and D34 compared with the acid group receiving water. The results indicated that duloxetine reversed anhedonic response of the acid group.
The acid group receiving pregabalin exhibited no significant difference throughout the recording (F(3,27)=2.098, p=0.124). The acid group receiving duloxetine had no difference at all timestamps compared with the vehicle group, but it exhibited significantly higher sucrose preference at D20, D27, and D34 compared with the acid group receiving water. The results suggested that pregabalin reduced anhedonic response of the acid group.
The acid group receiving diazepam exhibited no significant difference throughout the recording (F(3,27)=2.634, p=0.070). The acid group receiving diazepam showed significantly lower sucrose preference at D20 after starting drug administration compared with the vehicle group. Subsequently, the acid group treated with diazepam showed significantly higher sucrose preference at D27 and D34 compared with the acid group receiving water but no difference compared with the vehicle group. The data indicated that diazepam had delay reaction on anti-anhedonic response.
In addition to the percentage of sucrose preference, sucrose intake in the sucrose preference test revealed a similar trend as the sucrose preference did (Figure S2).
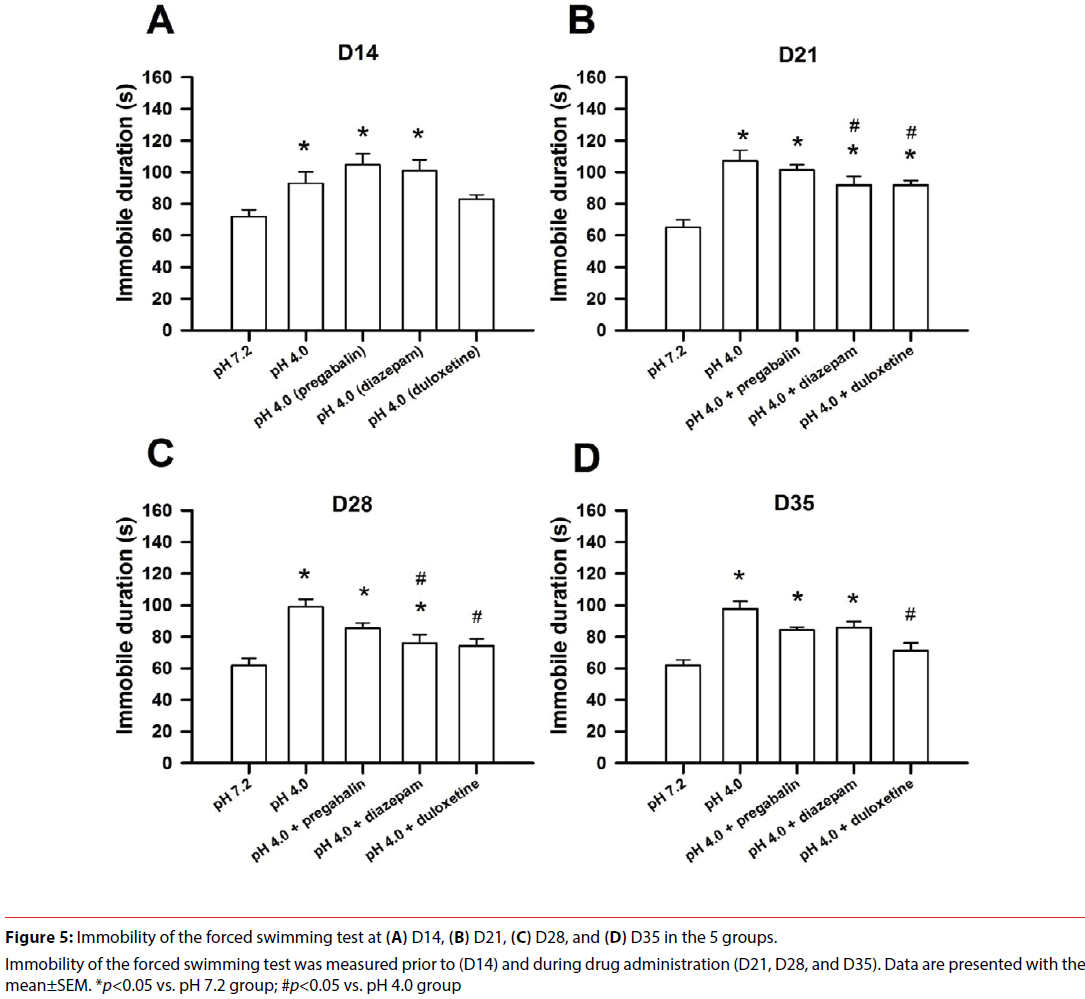
▪ Forced swimming test
No behavioural impediment was seen during the forced swimming test. Immobility of the forced swimming test at 4 testing time points in all groups is shown in Figure 5. Immobility had significant difference in the factors of treatment (F(4,135)=16.515, p<0.001), time (F(3,135)=12.349, p<0.001), and treatment×time (F(12,135)=2.163, p=0.017). There was no significant difference on the immobility throughout the recording period in the vehicle group (F(3,27)=2.188, p=0.113), which indicated no alteration in repeated measures during the forced swimming test. The acid group receiving water had no significant difference throughout the recording (F(3,27)=1.201, p=0.328). However, the acid group receiving water had significantly longer immobility at D14, D21, D28, and D35 compared with the vehicle group, which indicated development of despair-like behavior.
Figure 5: Immobility of the forced swimming test at (A) D14, (B) D21, (C) D28, and (D) D35 in the 5 groups.
Immobility of the forced swimming test was measured prior to (D14) and during drug administration (D21, D28, and D35). Data are presented with the mean±SEM. *p<0.05 vs. pH 7.2 group; #p<0.05 vs. pH 4.0 group Table
The acid group receiving duloxetine exhibited significant difference throughout the recording (F(3,27)=6.312, p=0.002). The acid group treated with duloxetine showed significantly longer immobility at D21 compared with the vehicle group, and it exhibited significantly shorter immobility at D21, D28, and D35 compared with the acid group receiving water. The results indicated that duloxetine had a delay action to reduce despair-like behavior of the acid group.
The acid group receiving pregabalin exhibited significant difference throughout the recording (F(3,27)=6.834, p=0.001). The acid group receiving pregabalin exhibited significantly longer immobility at D14, D21, D28, and D35 compared with the vehicle group, and it had no difference compared with the acid group receiving water. The results indicated that pregabalin didn’t modulate despair-like behavior.
The acid group receiving diazepam exhibited significant difference throughout the recording (F(3,27)=6.332, p=0.002). The acid group receiving diazepam showed significantly longer immobility at D14, D21, D28, and D35 compared with the vehicle group. This group had significantly shorter immobility at D21 and D28 compared with the acid group receiving water. The data indicated that diazepam had moderate remedy on despair-like behavior at early stage of administration.
Discussion
Major findings of this study are: 1) Repeated intramuscular injections of acidic saline elicited bilateral, long-lasting mechanical hyperalgesia, and comorbid anxio-depressive behavior. 2) Pregabalin significantly increased PWTs and ameliorated comorbidity of anxietylike and anhedonic behaviors. 3) Duloxetine significantly increased PWTs and ameliorated comorbidity of anhedonic and despair-like behavior. 4) Diazepam did not alter PWTs but ameliorated comorbidity of anxiety-like and anhedonic behavior. All results regarding various timepoints are summarized in Table 1. Our results indicate dissociation among these medicines in treatment of hyperalgesia and psychiatric comorbidity.
| group Day |
pH4.0 (water) |
pH4.0 (pregabalin) | pH4.0 (diazepam) |
pH4.0 (duloxetine) |
|---|---|---|---|---|
| 13-14 (W) | H+D | H+D | H+D | H |
| 20-21 (W/M) | H+DE | D | H+DE | H1+D |
| 27-28 (W/M) | H+ADE | D | H+D | A |
| 34-35 (W/M) | H+ADE | D | H+D | A |
1only contralateral hyperalgesia.
Table 1: Changes of hyperalgesia and psychiatric comorbidity in the 4 groups.
In the present study, repeated intramuscular injections of acidic solution elicited longlasting, bilateral, and mechanical hyperalgesia in 87% of rats, which is consistent with 72%- 90% of previous reports [10,12,16]. The acid-induced CWP model reveals widespread hyperalgesia with no muscle inflammation or pathology [25,26]. The present study observed acid-induced hyperalgesia accompanied by comorbidity of anxiety-like, despair-like and anhedonic behaviors [12]. The present study provides face validity of the acid-induced CWP model like clinical observations in FM patients [1,2].
Moreover, the present study systematically evaluated effects of three medicines on hyperalgesia and psychiatric comorbidity in the CWP model. Pregabalin and duloxetine, but not diazepam, significantly reduced acidinduced hyperalgesia through a chronic oral administration via gavage. The results not only extend our knowledge on short-term analgesic action of pregabalin [11] but also increase our understandings of these medicines on pain and psychiatric comorbidity in the acid-induced CWP model. Furthermore, these medicines selectively modulated anxiety-like behavior and subcategories (anhedonia or despair mood) of depression-like behavior, which is not fully reported in clinic [27,28]. Taken together, the present study strengthens face validity and predictive validity of the acid-induced CWP model as FM in humans.
The present study observed analgesic effect of pregabalin in the acid-induced CWP model [11]. To our knowledge, it is the first time to present anxiolytic response of pregabalin in this animal model. Pregabalin is a selective high-affinity ligand of the α2δ subunit of the voltage-dependent Ca2+ channels [29]. The potent binding of pregabalin at the α2δ site reduces calcium influx at nerve terminals, resulting in reduced release of numerous neurochemicals (including glutamate, norepinephreine, and substance P) [30-32]. This mechanism of action may be responsible for the anticonvulsant, analgesic, and anxiolytic activity of pregabalin in numerous animal studies [4]. The calcium channel α2δ sub unit expresses primarily in the amygdala, hippocampus and neocortex through immunostaining technique [33]. A recent study has indicated that activation of postsynaptic extracellular-regulated kinase (ERK)-dependent component in the central amygdala is required to development of the postsynaptic potentiation in the acid-induced muscle pain [14]. Infusion of pregabalin into the amygdala exhibits comparable analgesic effect as that observed via oral administration by gavage (unpublished results). Taken together, pregabalin probably activates calcium channels in the amygdaloid related pathway to produce analgesic or anxiolytic effect.
Pregabalin ameliorated anhedonic behavior in terms of high sucrose preference and sucrose intake but exhibited no effect on despair mood as indicated by long immobility of the forced swimming test. There is no clinical report dealing with pregabalin on subcategorical items of depressive symptom in FM patients. In a neuropathic pain model by partial sciatic nerve ligation, pregabalin reduces anhedonic phenomenon but has no change in immobility of the forced swimming test [34]. Pregabalin induces development of depression and/or suicide ideation in patients with persistent neuropathic pain [35]. The pregabalin results from neuropathic pain may support our findings even though there are large differences between these two pains in etiology and treatment. It is well known that anhedonia and despair of depression-like behavior involve different neural circuits [36]. Pregabalin may have different actions in these neural circuits.
In the present study, duloxetine (3 mg/kg, p.o.) significantly improved both hyperalgesia and depression-like behaviors of the acid-induced muscle pain. In a recent study, chronic muscle hyperalgesia was induced by biogenic amine depletion using reserpine and was attenuated by high dose duloxetine (30 mg/kg, p.o.) but not in low dose (3 or 10 mg/kg, p.o.) [22]. The discrepancy of effective duloxetine dose in these two muscle pain models may arise from different cellular mechanisms. For example, reserpine specifically causes dysfunction of all aminergic neurons which have widespread influence on the brain, whereas acid-induced CWP model activates specific acid-sensing ion channel (ASIC3) which results in altered activations of several brain regions [26]. Another possibility is the different testing periods, which can result in varied hyperalgesia. The present study assessed duloxetine continuously for 2 weeks after inducing hyperalgesia, which may indicate a long-lasting stable pain. In contrast, the biogenic amine depletion model only tests up to 4 hours after reserpine injection (i.e., acute phase).
The duloxetine-treated acid group (3 mg/kg, p.o.) exhibited anxiety-like behavior at D27 and D34, and the acid group also developed anxiety-like behavior at the same timepoints (Table 1). There is little guindance from clinical trials regarding the management of fibromyalgia and comorbid anxiety disorders. Both the duloxetine and pregabalin fibromyalgia trials have indicated that the reduction in pain is not related to alleviation of anxiety or depression symptom [5]. Recently, add-on therapy of pregabalin in the duloxetine treatment of fibromyalgia patients has been suggested to improve multiple clinical outcomes (including anxiety and sleep disturbance) [6]. A previous study using duloxetine (10 mg/kg, p.o.) shows no effect on the elevated plus maze test in a neuropathic pain model [37]. Duloxetine alone (10 mg/kg, i.p.) did not alter open arms entry frequency of an elevated plus maze in an anxious mice model [38]. In line with these results, duloxetine has little effect on the anxietylike behavior.
Diazepam, a long half-life benzodiazepamrelated compound, is a GABAA receptor agonist and exhibits both sedative and anxiolytic effects [39,40]. In this study, the acid group receiving diazepam still exhibited hyperalgesia, which extends understanding of acute diazepam treatment in the same model previously [16]. Bromazepam, a medium halflife benzodiazepam-related compound, exhibits negligible effect in fibromyalgia patients [41]. Taken together, these results indicate little analgesic effect of diazepam in the acid-induced CWP model and FM patients.
Diazepam is a classic anxiolytic, which has been demonstrated in numerous clinical studies [42] and various animal models with pain [40,43]. The present study also observed anxiolytic effect of diazepam in the acid-induced CWP model. Additionally, diazepam ameliorated anhedonic behavior progressively in terms of the sucrose preference test. Approaching the sucrose bottle could be a self-driven or self-motivating behavior. A previous study indicated the effect of diazepam on hedonic modulation as result of self-stimulation rather than the stimulationescape paradigm [44]. Moreover, diazepam increases intracranial self-stimulation behavior (i.e., reward enhancing effect) [45] and high risky decision making behavior [46]. In lines with these results, diazepam may have a new application on increased hedonia or positive reward in the CWP subjects.
In the present study, all anxiety- and depressionlike behaviors were repeatedly measured under the consideration of the 3R’s principle on animal use. To minimize possible errors of repeated measures, we performed all measures with a long inter-measure interval of a week, which is similar to those used in previous studies [22-24]. The vehicle group had no change in PWT, open-arms entry in the elevated plus maze, and immobility in the forced swimming test. A progressive increase sucrose preference and intake was found in the vehicle group, which indicate a pleasure seeking behavior as previous observation [19,47]. These data indicate that repetitive measures are appropriate under our design. However, the within-subject experimental design may raise a concern of historical consequence. To further characterize the effect of treatment on hyperalgesia or psychiatric comorbidity, studies with between-subject design are warranted.
Conclusion
Repeated intramuscular injections of acidic saline in rats elicited bilateral, long-lasting mechanical hyperalgesia and affective comorbidity in the acid-induced CMP model. Pregabalin, duloxetine, and diazepam exerted differently on hyperalgesia and anxio-depressive comorbidity. The current study provides additional face validity and predictive validity of the acidinduced CWP model as humans with CWP syndromes. Our results also suggest valuable information of these medicines in treatment of pain and affective comorbidity.
Acknowledgements
The authors thank Ms.Yu-Hsing Huang for her valuable support in the animal care. The authors appreciate valuable official support from the University of South Australia. This work was supported by the Ministry of Science and Technology (MOST103-2314-B-006-021-MY2 and MOST106-2420-H-006-003).
References
- Arnold LM, Hudson JI, Keck PE, et al. Comorbidity of fibromyalgia and psychiatric disorders. J. Clin. Psychiatry 67(8), 1219-1225 (2006).
- Epstein SA, Kay G, Clauw D, et al. Psychiatric disorders in patients with fibromyalgia. A multicenter investigation. Psychosomatics 40(1), 57-63 (1999).
- Panaszek B, Gomulka K, Wolanczyk-Medrala A, et al. Self-injurious behavior in fibromyalgia: a case report. Neuropsychiatry 7(2), 183-184 (2017).
- Bellato E, Marini E, Castoldi F, et al. Fibromyalgia syndrome: etiology, pathogenesis, diagnosis, and treatment. Pain. Res. Treat 2012(1), 426130 (2012).
- Arnold LM, Crofford LJ, Martin SA, et al. The effect of anxiety and depression on improvements in pain in a randomized, controlled trial of pregabalin for treatment of fibromyalgia. Pain. Med 8(8), 633-638 (2007).
- Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain 157(7), 1532-1540 (2016).
- Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert. Opin. Pharmacother 16(9), 1347-1368 (2015).
- Arnold LM. Management of psychiatric comorbidity in fibromyalgia. Curr. Psychiatry. Rep 8(3), 241-245 (2006).
- Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 338(1), 114-129 (2016).
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle. Nerve 24(1), 37-46 (2001).
- Yokoyama T, Maeda Y, Audette KM, et al. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J. Pain 8(5), 422-429 (2007).
- Liu YT, Shao YW, Yen CT, et al. Acid-induced hyperalgesia and anxio-depressive comorbidity in rats. Physiol. Behav 131(1), 105-110 (2014).
- Miranda A, Peles S, McLean PG, et al. Effects of the 5-HT3 receptor antagonist, alosetron, in a rat model of somatic and visceral hyperalgesia. Pain 126(1-3), 54-63 (2006).
- Cheng SJ, Chen CC, Yang HW, et al. Role of extracellular signal-regulated kinase in synaptic transmission and plasticity of a nociceptive input on capsular central amygdaloid neurons in normal and acid-induced muscle pain mice. J. Neurosci 31(6), 2258-2270 (2011).
- Sluka KA, Price MP, Breese NM, et al. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106(3), 229-239 (2003).
- Nielsen AN, Mathiesen C, Blackburn-Munro G. Pharmacological characterisation of acid-induced muscle allodynia in rats. Eur. J. Pharmacol 487(1-3), 93-103 (2004).
- Shaw FZ, Chuang SH, Shieh KR, et al. Depression- and anxiety-like behaviors of a rat model with absence epileptic discharges. Neuroscience 160(2), 382-393 (2009).
- Pellow S, Chopin P, File SE, et al. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 14(3), 149-167 (1985).
- Huang HY, Lee HW, Chen SD, et al. Lamotrigine ameliorates seizures and psychiatric comorbidity in a rat model of spontaneous absence epilepsy. Epilepsia 53(11), 2005-2014 (2012).
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev 29(4-5), 547-569 (2005).
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB. J 22(3), 659-661 (2008).
- Nagakura Y, Oe T, Aoki T, et al. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 146(1-2), 26-33 (2009).
- Suzuki T, Amata M, Sakaue G, et al. Experimental neuropathy in mice is associated with delayed behavioral changes related to anxiety and depression. Anesth. Analg 104(6), 1570-1577 (2007).
- Wang SH, Zhang ZJ, Guo YJ, et al. Anhedonia and activity deficits in rats: impact of post-stroke depression. J. Psychopharmacol 23(3), 295-304 (2009).
- Blackburn-Munro G. Pain-like behaviours in animals - how human are they? Trends. Pharmacol. Sci 25(6), 299-305 (2004).
- DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr. Pain. Headache. Rep 12(5), 338-343 (2008).
- Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain 136(3), 432-444 (2008).
- Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis. Rheum 50(9), 2974-2984 (2004).
- Bian F, Li Z, Offord J, et al. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain. Res 1075(1), 68-80 (2006).
- Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology 42(2), 229-236 (2002).
- Dooley DJ, Donovan CM, Meder WP, et al. Preferential action of gabapentin and pregabalin at P/Q-type voltage-sensitive calcium channels: inhibition of K+-evoked [3H]-norepinephrine release from rat neocortical slices. Synapse 45(3), 171-190 (2002).
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain 105(1-2), 133-141 (2003).
- Taylor CP, Garrido R. Immunostaining of rat brain, spinal cord, sensory neurons and skeletal muscle for calcium channel alpha2-delta (alpha2-delta) type 1 protein. Neuroscience 155(2), 510-521 (2008).
- La Porta C, Lara-Mayorga IM, Negrete R, et al. Effects of pregabalin on the nociceptive, emotional and cognitive manifestations of neuropathic pain in mice. Eur. J. Pain 20(9), 1454-1466 (2016).
- Hall TD, Shah S, Ng B, et al. Changes in mood, depression and suicidal ideation after commencing pregabalin for neuropathic pain. Aust. Fam. Physician 43(10), 705-708 (2014).
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat. Neurosci 13(10), 1161-1169 (2010).
- Gregoire S, Michaud V, Chapuy E, et al. Study of emotional and cognitive impairments in mononeuropathic rats: effect of duloxetine and gabapentin. Pain 153(8), 1657-1663 (2012).
- Patel S, Kale PP, Addepalli V, et al. Effect of a combination of duloxetine with hydroxyzine on experimental models of anxiety in mice. Ind. J. Pharmacol 47(2), 173-176 (2015).
- Sun GC, Hsu MC, Chia YY, et al. Effects of age and gender on intravenous midazolam premedication: a randomized double-blind study. Br. J. Anaesth 101(5), 632-639 (2008).
- Wallace VC, Segerdahl AR, Blackbeard J, et al. Anxiety-like behaviour is attenuated by gabapentin, morphine and diazepam in a rodent model of HIV anti-retroviral-associated neuropathic pain. Neurosci. Lett 448(1-9), 153-156 (2008).
- Quijada-Carrera J, Valenzuela-Castano A, Povedano-Gomez J, et al. Comparison of tenoxicam and bromazepan in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled trial. Pain 65(2-3), 221-225 (1996).
- Altamura AC, Moliterno D, Paletta S, et al. Understanding the pharmacokinetics of anxiolytic drugs. Expert. Opin. Drug Metab. Toxicol 9(4), 423-440 (2013).
- Parent AJ, Beaudet N, Beaudry H, et al. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav. Brain. Res 229(1), 160-167 (2012).
- Carden SE, Coons EE. Diazepam's impact on self-stimulation but not stimulation-escape suggests hedonic modulation. Behav. Neurosci 104(1), 56-61 (1990).
- Reynolds LM, Engin E, Tantillo G, et al. Differential roles of GABA(A) receptor subtypes in benzodiazepine-induced enhancement of brain-stimulation reward. Neuropsychopharmacology 37(11), 2531-2540 (2012).
- Mitchell MR, Vokes CM, Blankenship AL, et al. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology 218(4), 703-712 (2011).
- Chen SD, Wang YL, Liang SF, et al. Rapid amygdala kindling causes motor seizure and comorbidity of anxiety- and depression-like behaviors in rats. Front. Behav. Neurosci 10(1), 129 (2016).