Research Article - (2018) Volume 8, Issue 3
Long-Term Effects Of Combined Treatment With Memantine And Donepezil On Alzheimer's Disease Patients: 72-Week Study
- Corresponding Author:
- Rei Wake
Department of Psychiatry, Shimane University School of Medicine, 89-1 Enyacho, Izumo 693- 8501, Japan
Tel: +81-853-20-2262
Fax: +81-853-20-2260
Abstract
Abstract
Objective: In this study, we evaluated the long- term effects of memantine on cognitive function, BPSD, and the care burden in patients with moderate to severe Alzheimer’s disease (AD) for 72 weeks. Further, we examined the association between the long-term effects of memantine and brain blood flow using near-infrared spectroscopy (NIRS).
Methods: We evaluated the effects of memantine administration from baseline using the Clinical Global Impression - Improvement scale (CGI-I) , Mini-Mental State Examination (MMSE), Clock Drawing Test (CDT), Neuropsychiatric Inventory (NPI), Japanese version of the Zarit Burden Interview (J-ZBI), and NIRS on two groups; a combined donepezil and memantine administration group (combination group, n=19), and donepezil only administration group (control group, n=18) were assessed at weeks 0, 4, 12, 24, 48 and 72.
Results: A significant difference was found between the combination group and the control group in the scores of CGI-I after 4 weeks, CDT after 24 weeks, and NPI and J-ZBI after 12 weeks. A significant correlation was observed between the irritability subscore of NPI and total score of J-ZBI. In the NIRS measurements, there was no significant interaction in total value of Oxy- Hb integral from of all channels at the 72nd week, although oxyhemoglobin suppression was observed in CH5, CH7, and CH8 at 12th and 24th week.
Conclusions: In this study, long-term administration of memantine to AD patients improved clinical symptoms overall, cognitive function, and BPSD, thereby reducing the burden of caregivers and inhibited transiently the reduction of cerebral blood flow in the prefrontal area.
Keywords
Alzheimer’s disease, Memantine, Donepezil, Near-infrared spectroscopy (NIRS), Long-term effects
Introduction
Alzheimer disease (AD) is the most prevalent type of dementia, accounting for more than 80% of cases of dementia in middle-aged and old patients [1]. AD is a neurodegenerative disorder characterized by behavioral and psychological symptoms of dementia (BPSD), such as agitation, irritability and wandering [2]. This disease affects the memory, judgment, and reason, mainly due to the loss of neurons and their synapses in the cerebral cortex and hippocampus [3]. The cognitive impairment in AD is correlated with a significant reduction in the cholinergic activity [4]. This disease poses an extremely heavy physical and mental burden on the patient and his or her family.
Current treatment strategies focus primarily on medications and are aimed at alleviating symptoms. The following four drugs are approved for the treatment of Alzheimer’s disease worldwide: memantine and three cholinesterase inhibitors (donepezil, galantamine, and rivastigmine) [5]. Cholinesterase inhibitors (ChEIs) and N-methyl D-aspartate (NMDA) receptor antagonists are the two most prevalent types of medicine approved by the U.S. Food and Drug Administration. When the metabolizing enzyme is suppressed, acetylcholine (Ach) activity is increased, and cognitive functions improve in turn [6]. In addition, NMDA receptor antagonists regulate glutamatergic neurons activities that facilitate synaptic plasticity and neuronal growth and differentiation, thereby enhancing cognition, learning, and memory [1,7].
Numerous studies have investigated the effects of the aforementioned medicines on cognitive functions and BPSD in patients with AD. Patients with moderate to severe AD exhibit relatively severe cognitive and psychological symptoms. ChEIs and NMDA remain the main treatments. Donepezil is the most common ChEI used for AD treatment, and memantine is the most prevalent choice among NMDAs. Memantine has been approved worldwide for treating moderate-to-severe Alzheimer’s disease. It is postulated that memantine exerts its therapeutic effect through its action as a low-to-moderate affinity, noncompetitive (open channel), nonselective, voltage-dependent, N-methyl- D-aspartic acid (NMDA) receptor antagonist, which binds preferentially to NMDA receptoroperated calcium channels5. Memantine blocks the effects of sustained, pathologically elevated levels of glutamate, which could otherwise lead to neuronal dysfunction [8-10]. In addition, memantine may also upregulate NMDA receptor expression, causing activation in the presence of a strong stimulus [11].
The combination of memantine and donepezil can improve AD symptoms through their different mechanisms [12-14]. Despite the wealth of information on the ChEIs and memantine for treating AD, the magnitude of the effects of administering of donepezil or a combination of memantine and donepezil on patients’ cognitive functions, BPSD, and global functions remains unclear.
Previous studies have also indicated that when the course of the disease progresses to moderate or severe levels, combination treatments are more effective than single treatment for delaying the degradation of cognitive functions [12,14,15-22]. Further, we have reported that memantine administered to patients with moderate to severe AD may inhibit the reduction of cerebral blood flow in the prefrontal area, improve global clinical symptoms, cognitive function and BPSD, and lighten the burden of care placed on caretakers [23]. Although each medication has the effect of improving cognitive function for a certain period and delaying cognitive dysfunction, they still cannot treat the clinical condition for a long time. This makes the early identification and management of AD of great importance. However, there are few studies on the effects of long-term memantine administration on the burden of care, and there are no reports on this subject. Moreover, the association between NIRS findings and the therapeutic effects of anti-dementia drugs has been little studied. In addition, no objective AD diagnosis method has yet been established in other functional brain imaging studies.
In this study, we evaluated the long-term effects and relationship of memantine to cognitive function, BPSD, and the care burden in moderate to severe AD patients being treated with donepezil. We also closely examined the relationship between memantine’s long-term effects and cerebral blood flow using NIRS.
Object
▪ Subjects
The subjects comprised 37 patients with moderate to severe AD (mean age 78.8 ± 7.7 years), who are outpatients being treated at the Department of Clinical Psychiatry and Neurology, Shimane University Hospital. They had been diagnosed with AD using the diagnostic criteria set out in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) and the International Classification of Diseases, 10th edition (ICD-10), scoring 3 to 16 points on Hasegawa’s Dementia Scale-Revised (HDS-R), and had been treated with donepezil for 6 months or longer. The subjects were divided randomly, using a random number table into two groups: a memantine combinated with donepezil group (Combination group, n= 19, 77.9 ± 9.8 years) and a donepezil group without memantine (Control group, n = 18, 79.8 ± 4.6 years) (Table 1).
| Combination group | Control group | Total | p value *1 | ||
|---|---|---|---|---|---|
| Number of cases | 19 | 18 | 37 | - | |
| Gender | Male Female |
11 8 |
7 11 |
18 19 |
0.26 |
| Age (years) | Mean ± standard deviation | 77.9 ± 9.8 | 79.8 ± 4.6 | 78.8 ± 7.7 | 0.45 |
| HDS-R at enrolment (points) | Mean ± standard deviation | 10.5 ± 3.5 | 12.3 ± 3.0 | 10.9 ± 3.6 | 0.10 |
Table 1: Baseline subject characteristics.
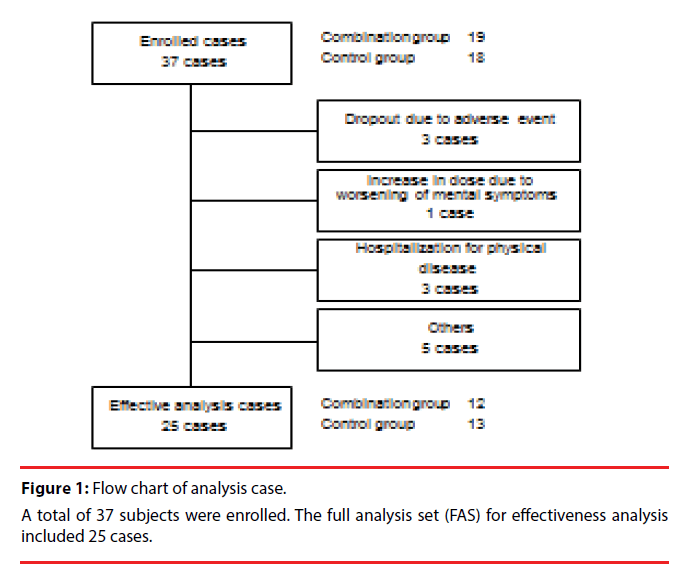
Thirty seven patients with moderate to severe AD (mean age of 78.8 ± 7.7 years) undergoing treatment with donepezil for over six months were divided into two groups: the memantine plus donepezil group (Combination group, n = 19, 77.9 ± 9.8 years old) and the donepezil alone group (Control group, n = 18, 79.8 ± 4.6 years old).
This study was conducted after review by and approval of the Ethics Committee of Shimane University’s Faculty of Medicine. Patients and their families received an explanation in writing of the purpose of the study and the content of the tests beforehand and signed a consent form to agree to participation.
▪ Measurement methods
The Combination group continued donepezil treatment and took oral memantine for 72 weeks. Memantine administration started at 5 mg/day, and the dose was increased by 5 mg every week until reaching the maintenance dose of 20 mg/day. The Clinical Global Impression - Improvement scale (CGI-I), Mini-Mental State Examination (MMSE), Clock Drawing Test (CDT), Neuropsychiatric Inventory (NPI), Japanese version of the Zarit Burden Interview (J-ZBI) were given and brain blood flow was measured by near-infrared spectroscopy (NIRS) six times: at enrollment, 4 weeks, 12 weeks, 24 weeks, 48 weeks, and 72 weeks after enrollment.
▪ Primary outcome measures
The Clinical Global Impression - Improvement scale (CGI-I) evaluates the improvement in the patient’s condition from before intervention on a scale of one to seven: 1-greatly improved, 2-improved, 3-slightly improved, 4-unchanged, 5-slightly deteriorated, 6-deteriorated, and 7-greatly deteriorated. The Mini-Mental State Examination (MMSE) is a screening test for dementia developed by Folstein et al. [24] It consists of 11 questions to evaluate orientation, memorization, calculating ability, linguistic competence and graphic competence with a perfect score of 30 points. The Clock Drawing Test (CDT) scores the dial face and clock hands drawn by the subject: it is used as simple screening test for dementia. In the past, it was thought to reflect the function of the parietal lobes [25]. However, it is now thought to test for a wide range of cognitive functions, including executive function. This study adopted the method of drawing a clock on a sheet of blank paper according to verbal instruction because it is considered to best reflect frontal lobe function [26]. Freedman’s method [27] was used for rating. The Neuropsychiatric Inventory (NPI) is based on a structured interview with a caretaker who is fully familiar with the patient’s behavior, such as a spouse. NPI is highly regarded as a mental symptom assessment scale for dementia patients, and is frequently used as an indicator to evaluate the effects of anti-AD drugs in clinical trials. The Zarit Burden Interview (J-ZBI) formulated by Steven Zarit at Pennsylvania State University is one of the most common scales for evaluating the burden of care in the world [28]. It is a scale that totals the physical psychological and economic burdens created by nursing care and measures it as an overall care burden. This study used the J-ZBI prepared by a group at the National Center for Geriatrics and Gerontology [29].
▪ NIRS
NIRS is a brain function measuring technique using near-infrared light. It is increasingly being employed in clinical applications due to its greater ease of use than other brain function measuring techniques. Measurements can be taken with the patient in any posture or even while in motion. to quantify brain activity status, changes in oxygenated hemoglobin [Oxy- Hb] and deoxygenated hemoglobin [Deoxy-Hb] concentrations, both of which are dependent on local cerebral blood flow changes induced by cerebrocortical neuron activities, are measured by exposing the head to near-infrared light and measuring the reflected light. It enables noninvasive and easy viewing of changes in blood flow in the cerebral cortex.
This study adopted the standard procedure for NIRS established by Takizawa et al. [30] Using an ETG-4000 (Hitachi Medical, Tokyo, Japan), the bases of the measurement probes for the frontal region were placed at positions Fp1 and Fp2 (International 10-20 system). With the subject wearing a 22-channel probe on the frontal region, changes in the hemoglobin contentration of the blood in the cerebral cortex were measured while performing a verbal fluency task, which is a frontal cortex-activating task. As an activation task, each subject was instructed to say as many words as they could come up with, starting with the letter spoken by the tester. The first letters were changed every 20 seconds, and the activation task continued for 60 seconds. Before and after the activation task, the subjects were instructed to repeat “A, I, U, E, O” aloud to establish the baseline condition.
▪ Statistical analysis
To examine the effects of memantine on the test results, two-way repeated-measures ANOVA was performed on the changes in the scores of the Combination and Control groups (changes in values of the integral of the results of NIRS measurement) at enrollment, 4, 12, 24, 48, and 72 weeks. To examine in detail the differences between the groups, a multiple comparison using Bonferroni’s method was performed. Channels including artifacts and noise (low S/N) due to body motion were excluded from the results of NIRS measurements, and inter-channel influence was corrected using the false discovery rate (FDR) method (two-tailed; we set the value of q specifying the maximum false discovery rate to 0.05, so that there are no more than 5% false positives on average) when we performed 22 paired t-tests. For each endpoint of effectiveness and safety, the significance level of the two-sided test was set to 0.05. We then compared the two groups using Student’s t-test, and the significance level in the analysis was set to P = 0.05. The Pearson correlation coefficient was used to examine the relationship between the tests.
Result
There were 37 patients enrolled in total. The full analysis set (FAS) for efficacy analysis included 25 cases, excluding the patients as shown in Figure 1. The individual reasons for exclusion are listed in Figure 1. Change from the baseline for each endpoint is shown in Table 2.
| Endpoint | Change from the baseline (72 weeks later) | ||||
|---|---|---|---|---|---|
| Combination group (n = 12) |
Control group (n = 13) |
p value *1 | |||
| Mean | SD | Mean | SD | ||
| CGI-I | 0.43 | 0.30 | 2.15 | 1.07 | <0.001*** |
| MMSE | -0.83 | 3.41 | -4.38 | 3.73 | 0.019 |
| CDT | 0.67 | 1.11 | -4.00 | 3.84 | 0.003** |
| NPI | 1.70 | 5.80 | 39.3 | 28.6 | <0.001*** |
| J-ZBI | -0.25 | 1.66 | 1.54 | 1.39 | 0.008** |
| NIRS (Mean of all channels) |
7.85 | 46.34 | -30.12 | 45.35 | 0.643 |
**: p<0.01, ***: p<0.001
**: p<0.01, ***: p<0.001: Change from the baseline for each endpoint is shown.
Table 2: Change from the baseline and results of analysis of variance.
Effects
▪ Combinational effect of memantine on global symptoms and cognitive function
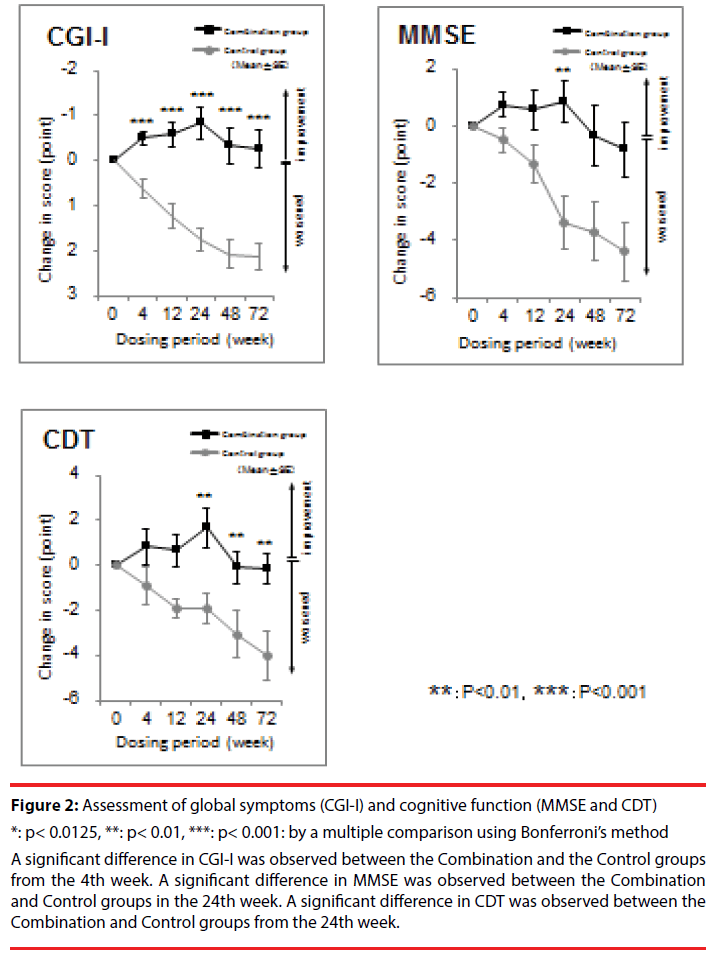
In the evaluation of global symptoms, there was a significant interaction between the Control and Combination groups in CGI-I (F = 37.67 (1,22), p < 0.001). Regarding cognitive function, there was significant interaction between the Combination group and the Control group in MMSE (F = 9.39 (1,22), p = 0.01) and in CDT (F = 15.84 (1,22), p < 0.001) (Figure 2).
Figure 2: Assessment of global symptoms (CGI-I) and cognitive function (MMSE and CDT)
*: p< 0.0125, **: p< 0.01, ***: p< 0.001: by a multiple comparison using Bonferroni’s method
A significant difference in CGI-I was observed between the Combination and the Control groups from the 4th week. A significant difference in MMSE was observed between the Combination and Control groups in the 24th week. A significant difference in CDT was observed between the Combination and Control groups from the 24th week.
▪ Combinational effect of memantine on BPSD
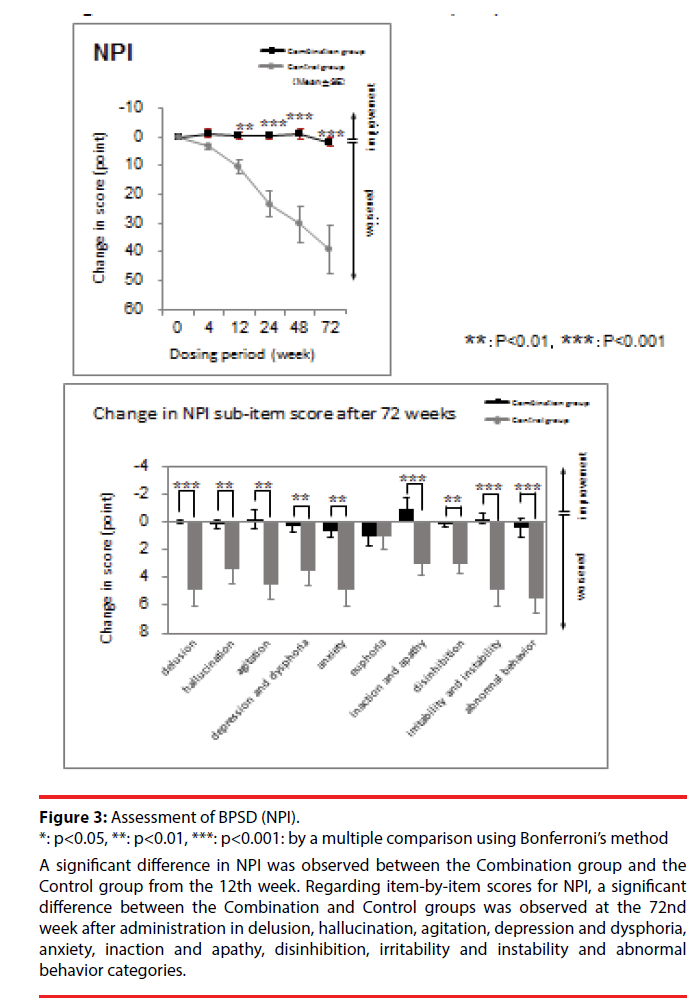
Regarding BPSD assessment, there was significant interaction between the Combination and Control groups in NPI (F = 47.76 (1,22), p < 0.001). A significant difference between the Combination and Control groups in the item-by-item NPI score was observed in delusion, hallucination, agitation, depression and dysphoria, anxiety, inaction and apathy, disinhibition, irritability and instability, and abnormal behavior (Figure 3).
Figure 3: Assessment of BPSD (NPI).
*: p<0.05, **: p<0.01, ***: p<0.001: by a multiple comparison using Bonferroni’s method
A significant difference in NPI was observed between the Combination group and the Control group from the 12th week. Regarding item-by-item scores for NPI, a significant difference between the Combination and Control groups was observed at the 72nd week after administration in delusion, hallucination, agitation, depression and dysphoria, anxiety, inaction and apathy, disinhibition, irritability and instability and abnormal behavior categories.
▪ Combinational effect on care burden
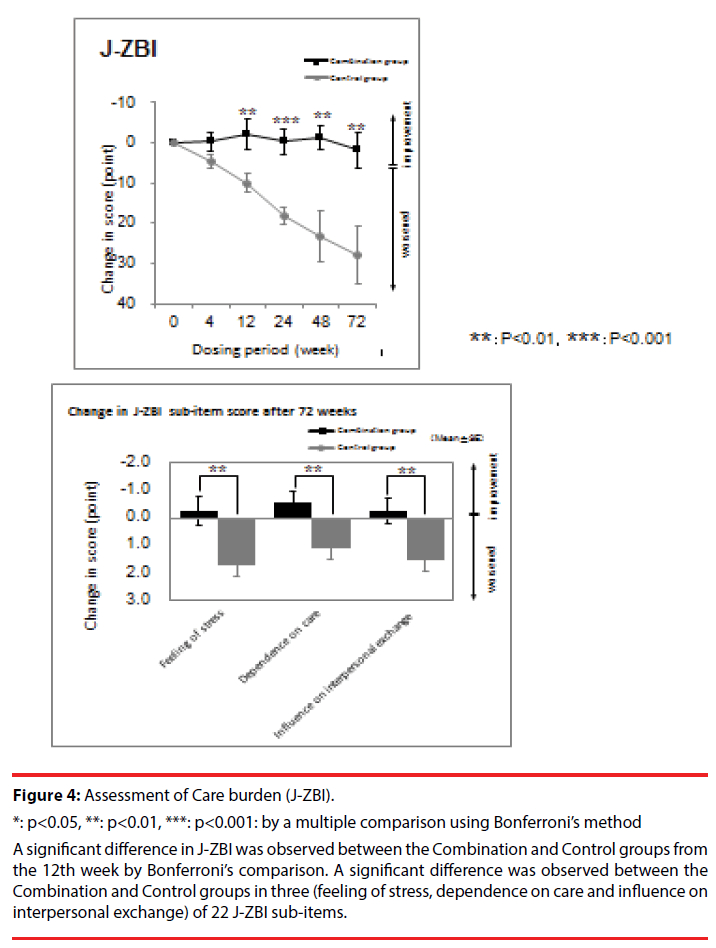
In the care burden assessment, there was significant interaction between the Combination and Control groups in J-ZBI (F = 18.91 (1,22), p < 0.001). A significant difference was observed in 3 out of 22 sub-items of J-ZBI (Figure 4).
Figure 4: Assessment of Care burden (J-ZBI).
*: p<0.05, **: p<0.01, ***: p<0.001: by a multiple comparison using Bonferroni’s method
A significant difference in J-ZBI was observed between the Combination and Control groups from the 12th week by Bonferroni’s comparison. A significant difference was observed between the Combination and Control groups in three (feeling of stress, dependence on care and influence on interpersonal exchange) of 22 J-ZBI sub-items.
▪ Change in NIRS measurements
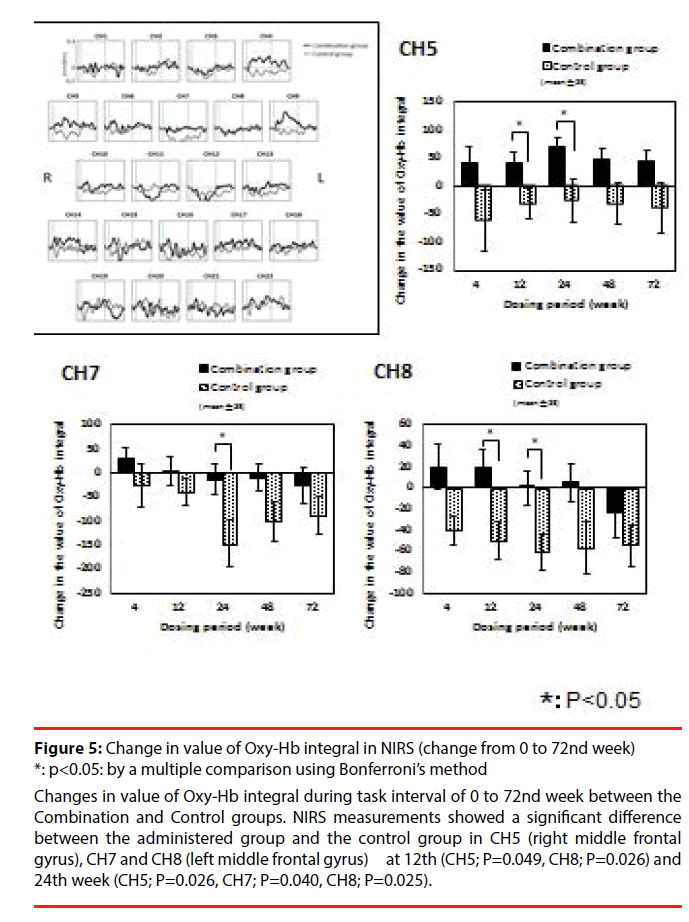
Regarding NIRS measurement, change in value of Oxy-Hb integral from week 0 was compared between the Combination group and the Control group. There was no significant interaction in total value of Oxy-Hb integral from of all channels. A significant difference between the administered group and the control group was observed at the 12th (CH5; P=0.049, CH8; P=0.026) and 24th week (CH5; P=0.026, CH7; P=0.040, CH8; P=0.025) in CH5 (right middle frontal gyrus), CH7 and CH8 (left middle frontal gyrus) (t-test). But there was no significant interaction in total value of Oxy-Hb integral from of all channels at the 48th week (Figure 5).
Figure 5: Change in value of Oxy-Hb integral in NIRS (change from 0 to 72nd week) *: p<0.05: by a multiple comparison using Bonferroni’s method
Changes in value of Oxy-Hb integral during task interval of 0 to 72nd week between the Combination and Control groups. NIRS measurements showed a significant difference between the administered group and the control group in CH5 (right middle frontal gyrus), CH7 and CH8 (left middle frontal gyrus) at 12th (CH5; P=0.049, CH8; P=0.026) and 24th week (CH5; P=0.026, CH7; P=0.040, CH8; P=0.025).
▪ Relationship among measurements
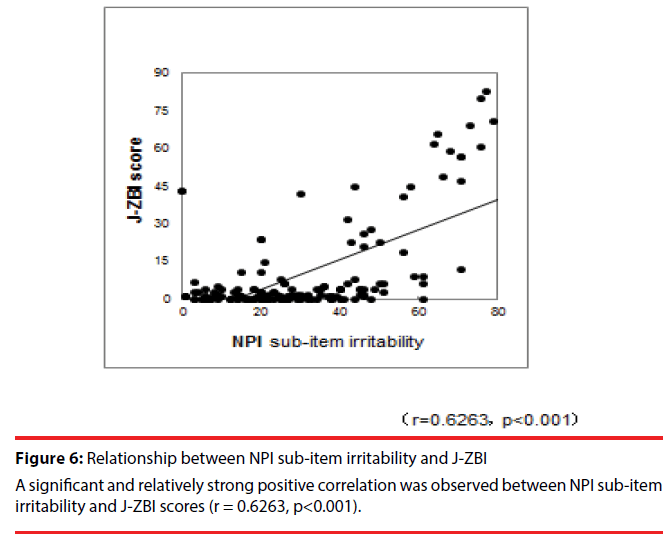
A study of the relationships among the test results revealed a significant and relatively strong positive correlation between NPI sub-item irritability and J-ZBI scores (r = 0.6263, p< 0.001) (Figure 6).
▪ Safety
Of the adverse events that occurred during the study, a causal relationship with memantine could not be ruled out in three cases and thus led to discontinuation of the drug. The most frequent adverse event was gait instability (2 cases, 10.5% of the Combination group).
In laboratory data, alanine aminotransferase (ALT) was elevated from 14 to 51 in one case in the Control group. Its relationship to this study is not known. There were no other abnormal values.
Discussion
We evaluated the long-term efficacy of memantine when given concomitantly with the cholinesterase inhibitor donepezil by use of rating scales for cognitive function, BPSD, and the care burden. Our previous finding was that at the endpoint or in the 24th week of treatment for moderate to severe AD, the combination treatment group exhibited greater improvement in cognitive functions, BPSD, and global functions than did the control group. In this study, the most important finding of the present study was that at the endpoint, the 72nd week, of treatment for moderate to severe AD, the combination treatment group exhibited greater improvement in cognitive functions, BPSD, and global functions than did the control group, though a significant difference in MMSE scores was not observed.
Memantine is a noncompetitive receptor antagonist, and it delays the process of dementia by preventing the pathological activation of NMDA receptors. In a cognitive function assessment, significant differences in CDT were observed between the Combination group and the Control group from the 24th week, respectively. CDT-command requires multiple cognitive domains, such as receptive language, memory, abstraction, and executive functions [31,32]. Memantine appears to inhibit overactivation of NMDA receptors, thus protecting neurocytes and limiting memory loss and learning disorders. Its neuroprotective effects have been observed in various neurological disorders. Importantly, memantine reportedly reduces the release of proinflammatory factors in activated microglia [33,34]. Major neuropathological changes in AD are neuritic plaque and neurofibrillary tangle (NFT), and it has been pointed out that NFT is related to the occurrence of psychological symptoms [35]. Because memantine inhibits abnormal phosphorylation of tau protein, it is thought to prevent formation of NFTs and thus reduce mental symptoms and to be effective against BPSD. In the assessment of global symptoms, a significant difference between the Combination and the Control groups in the CGI-I score was observed from the fourth week. In other words, worsening of global symptoms could be more significantly ameliorated by these effects.
Memantine is often used in combination with cholinesterase inhibitors because they have entirely different mechanisms of action. Our studies showed that memantine exhibited significant efficacy compared to controls in improving delusion, agitation/aggression, disinhibition, and nighttime disturbance/ diurnal rhythm disturbances in patients with AD. Moreover, memantine seems to facilitate the treatment of hallucination and irritability/ lability. These symptoms are classified as positive symptoms. Memantine was similar to controls for treatment of negative symptoms, such as dysphoria, euphoria, aberrant motor activity/ activity disturbances, and eating disturbances. Memantine improves cognitive functions36 and anti-dementia drugs may prevent brain atrophy in patients with AD [21].
A significant difference in changes over time of the NPI score used to assess BPSD was observed between the Combination and Control groups from the 12th week. In the item-by-item NPI score, a significant difference between the Combination and Control groups was observed at the 24th week after administration in delusion, agitation, depression and dysphoria, anxiety, inaction and apathy, irritability and instability and abnormal behavior.
Further, a significant difference in the J-ZBI score for assessing the care burden was observed between the Combination and Control groups from the 12th week. Significant differences from the Control group in item-by-item scores were observed in the following items: feeling of stress, dependence on care and influence on interpersonal exchange. It has been pointed out that BPSD sometimes causes family dysfunction and damages home nursing care [36,37]. In addition, the correlation found between NPI and J-ZBI in this study suggests that reduced BPSD leads to a lower care burden. Based on these findings, it appears that dementia medications such as memantine lighten the burden of care due to improvement of cognitive function, increased activity, and stabilization of emotions.
As regards cerebral blood flow in frontal cortex, a significant inhibition of reduction of cerebral blood flow was observed in bilateral middle frontal gyrus from 12 weeks to 24 weeks. The previous fMRI study observed strong deactivation in the medial frontal cortex which suggests that memory encoding of novel faces requires an increase in attentions [38]. We might infer from those findings that a slower decline in cognitive function results in a lighter care burden.
A review of published clinical trials of memantine indicated that early treatment of hypertension, a risk factor for stroke, reduces the risk of vascular dementia and slows its progression [39]. In addition, a previous study showed that memantine may be effective in improving the memory of patients with MCI [40]. As memantine may inhibit oxidative stress and inflammation during the early stage of the disease, memantine therapy as an anti-dementia drug could be effective without co-adiministration of any of the drug treatments for AD. But the results of the meta-analysis indicate that the use of the combination resulted in a larger effect size than the use of a ChEI alone in BPSD treatment22. Consequently, the optimal treatment effect on BPSD can be achieved through the combination of memantine and donepezil.
In our study, there were no serious side effects to stop using memantine for 72 weeks. Long-term studies show good tolerability of memantine with an acceptable side-effect profile [41]. It must be concluded that memantine is a relatively safe drug with few side effects and neglectable risk of drug-drug interactions, but adverse drug reactions (ADRs) of memantine (Stop using memantine serious side effects: cough, chest tightness, fever, trouble breathing; chest pain, fast heart rate; confusion, hallucinations; sudden numbness or weakness, especially on one side of the body; lack of coordination; fainting or seizure (convulsions); urinating less than usual or not at) are found in clinical trials in mild to severe dementia [42]. The effectiveness and side effects of memantine might be determined by the hepatic clearance of caffeine or various drugs. The metabolism might have accelerated with relevance to the current global NAFLD epidemic by maintaining connections between the brain and the adipose tissue-liver crosstalk [43-45].
As this study suggests, the effects of combination therapy in memantine and donepezil may continue for a relatively long time.
Limitations
The results of this study must be viewed in light of some limitations. Because the analysis was based on cross-sectional data, causality cannot be determined. Further, we should pay attention to the clinical importance corresponding to the statistical significance. Thus, more longitudinal studies are needed to assess cause-and-effect relationships. It is possible, however, that differences in assessment procedures and the small number of patients in this study may have influenced the results. Further studies with larger numbers of patients are required.
Conclusion
In this study, long-term administration of memantine to moderate to severe AD patients improved cognitive functions, BPSD, and global functions. The results of this study suggest that memantine administered to patients with moderate to severe AD could inhibit the reduction of cerebral blood flow in the left superior temporal lobe. The next step will be to conduct a large-scale study of the clinical characteristics of memantine as an anti-dementia drug with an increased number of cases.
Conflict of interest
None declared.
Acknowledgement
The authors declare that there are no conflicts of interest in relation to the subject of this study. Funding came from a Junior Scientist from the Shimane University Faculty of Medicine, and Health and Labour Sciences Research Grants, Japan.
Author Contributions
W.R., M.T. designed the study. H.S., H.J. coordinated the study. A.T. analyzed the data. W.R., M.T., H.J., N.M., F.M., and H.M., collected the clinical and pathological information for the AD cases. W.R., M.T. actively worked on the design, drafting and revision of the manuscript.
Keypoints
In this study, we evaluated the long-term effect2 of memantine on cognitive function, BPSD and the care burden, in patients with moderate-tosevere Alzheimer’s disease (AD).
In addition, we examined the association between the effects of memantine on brain blood flow using near-infrared spectroscopy (NIRS).
Administering memantine to AD patients was suggestesd to improve clinical symptoms overall, cognitive function, and BPSD, thereby reducing the care burden of caregivers.
Moreover, the reduction of cerebral blood flow in the prefrontal area might to be inhibited transiently during the long-term administration of memantine was observed.
References
- Kumar A, Singh A, Ekavali. A review on Alzheimer's disease pathophysiology and its management: An update. Pharmacol. Rep 67(2), 195–203 2015.
- Kristensen M. Neurotransmitters in Alzheimer's disease. Ugeskr. Laeger. 152(30), 2165-2168 (1990).
- Resende R, Pereira C, Agostinho P. et al. Susceptibility of hippocampal neurons to A peptide toxicity is associated with perturbation of Ca 2t homeostasis. Brain. Res 11(1), 11-21 (2007).
- Francis PT, Palmer AM, Snape M, et al. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J. Neurol. Neurosurg. Psychiatry 66(1), 137-147 (1999).
- Nakamura Y, Kitamura S, Nagakubo T, et al. Study of memantine hydrochloride in combination with donepezil hydrochloride in patients with moderate to severe Alzheimer’s disease – Efficacy and safety. Japanese. J. of Geri. Medi 54(1), 1147-1158 (2016).
- Ehret MJ, Chamberlin KW. Current practices in the treatment of Alzheimer disease: Where is the evidence after the phase III trials? Clin. Ther 37(8), 1604-1616 (2015).
- Deardorff WJ, Feen E, Grossberg GT. The use of cholinesterase inhibitors across all stages of Alzheimer's disease. Drugs. Aging 32(7), 537-547 (2015).
- Forest Laboratories Evaluation An of the safety and efficacy of memantine in agitated patients with moderate to severe Alzheimer’s disease. ClinicalTrials.gov (2004).
- Florida Atlantic University Delaying the progression of driving impairment in individuals with mild Alzheimer’s disease (2007).
- Washington University School of Medicine Corticolimbic Degeneration and Treatment of Dementia. ClinicalTrials.gov (2008).
- New York University School of Medicine Effects of memantine on magnetic resonance (MR) spectroscopy in subjects at risk for Alzheimer’s disease. ClinicalTrials.gov (2009).
- Campos C, Rocha NBF, Vieira RT, et al. Treatment of cognitive deficits in Alzheimer's disease: A psychopharmacological review. Psychiatria. Danubina 28(1), 2-12 (2016).
- Riverol M, Slachevsky A, López OL. Efficacy and tolerability of a combination treatment of memantine and donepezil for Alzheimer's disease: A literature review evidence. Eur. Neurol. J 3(1), 15-9 (2011).
- Tsoi KK, Chan JY, Leung NW, et al. Combination therapy showed limited superiority over monotherapy for Alzheimer disease: A meta-analysis of 14 randomized trials. J. Am. Med. Dir. Assoc 17(9), 863 e1-8 (2016).
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer's disease. N. Engl. J. Med 366(10), 893–903 (2012)..
- Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane. Database. of .Syst. Rev.. 25(1), CD001190 (2006).
- Di Santo SG, Prinelli F, Adorni F, et al. A meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer's disease. J. Alzheimers. Dis 35(2), 349–361 (2013).
- Molino I, Colucci L, Fasanaro AM, et al. Efficacy of memantine, donepezil, or their association in moderate-severe Alzheimer's disease: a review of clinical trials. Scientific. World. Journal (2013).
- Tan CC, Yu JT, Wang HF, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. J. Alzheimers Dis 41(2), 615–31 (2014).
- Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer's disease: a systematic review. BMJ. Open 2(3), e000917 (2012).
- Kishi T, Matsunaga S, Iwata N. The effects of memantine on behavioral disturbances in patients with Alzheimer's disease: a meta-analysis. Neuropsychiatr. Dis. Treat 13(1), 1909-1928 (2017).
- Chen R, Chan PT, Chu H, et al. Treatment effects between monotherapy of donepezil versus combination with memantine for Alzheimer disease: A meta-analysis. PLoS. One 12(8), e0183586 (2017).
- Araki T, Wake R, Miyaoka T, et al. The effects of combined treatment of memantine and donepezil on Alzheimer's disease patients and its relationship with cerebral blood flow in the prefrontal area. Int. J. Geriatr. Psychiatry 29(9), 881-889 (2014).
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12(1), 189-98 (1975).
- Tuokko H, Hadjistavropoulos T, Miller JA, et al. The Clock Test: a sensitive measure to differentiate normal elderly from those with Alzheimer disease. J. Am. Geriatr. Soc 40(1), 579-84 (1992).
- Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J. Neurol. Neurosurg. Psychiatry. 64(1), 588-94 (1998).
- Freedman M, Leach L, Kaplan E, et al. Clock Drawing: A Neuropsychological Analysis. Oxford University Press (1994).
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 20(1), 649-655 (1980).
- Arai Y, Kudo K, Hosokawa T, Washio M, Miura H, and Hisamichi S. Reliability and validity of the Japanese version of the Zarit Caregiver Burden interview. Psychiatry Clin. Neurosci. 51(1), 281-287 (1997).
- Takizawa R, Kasai K, Kawakubo Y, et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr. Res 99(1), 250-262 (2008).
- Matsunaga S, Kishi T, Iwata N. Combination therapy with cholinesterase inhibitors and memantine for Alzheimer’s disease: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol 18(5), (2014)
- Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and psy¬chological symptoms of dementia. Front. Neurol 3(1), 73 (2012).
- Cooper C, Li R, Lyketsos C, et al. Treatment for mild cognitive impairment: systematic review. Br. J. Psychiatry 203(1), 255-264 (2013).
- Wang F, Zou Z, Gong Y, et al. Regulation of human brain microvascular endothelial cell adhesion and barrier functions by memantine. J. Mol. Neurosci 62(1), 123-129 (2017).
- Farber NB, Rubin EH, Newcomer JW, et al. 2000. Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Arch. Gen. Psychiatry 57(1), 1165-1173 (2000).
- Kitamura S, Homma A, Nakamura Y, et al. Phase II study of memantine hydrochloride, a new NMDA receptor antagonist, in patients with moderate to severe Alzheimer’s disease – Efficacy, safety and recommended dose. Japanese. Journal. of. Geriatric. Psychiatry. 22(1), 453-463 (2011).
- Asada T. Analysis of breakdown in family care for patients with dementia. Psychiatr. Neurol. Jpn 93(1), 403-33 (1991).
- Rombouts, SARB, Barkhof F, et al. Altered Resting State Networks in Mild Cognitive Impairment and Mild Alzheimer’s Disease: An fMRI Study 239(1), 231-239 (2005).
- Winblad B, Jones RW, Wirth Y, et al. Memantine in moderate to severe Alzheimer’s disease: a meta-analysis of randomized clinical trials. Dement. Geriatr. Cognition. Disorders 24(1), 20-27 (2007).
- Ilhan AD, Dagli AS, Ozkan S, et al. Memantine improves semantic memory in patients with amnestic mild cognitive impairment: A single-photon emission computed tomography study. J. Mol. Neurosci 62(1), 123-129 (2017).
- Robinson DM, Keating GM. Memantine: a review of its use in Alzheimer's disease. Drugs 66(11), 1515-1534 (2006).
- Robert J van Marum. Update on the use of memantine in Alzheimer's disease. Neuropsychiatr. Dis. Treat.; 5(1), 237-247 (2009).
- Martins IJ. Drug Therapy for Obesity with Anti-Aging Genes Modification. Ann. Obes. Disord 1(1), 1001 (2016).
- Martins IJ. Diet and Nutrition reverse Type 3 Diabetes and Accelerated Aging linked to Global chronic diseases. J. Diab. Res. Ther 2(2), 2016.
- Martins IJ. Caffeine with Links to NAFLD and Accelerated Brain Aging. Chapter: Non-Alcoholic Fatty Liver Disease - Molecular Bases, Prevention and Treatment. InTech - Open Science Open Minds | InTechOpen