Case Report - Neuropsychiatry (2023) Volume 13, Issue 3
Intramedullary Secondary Spinal Cord Lymphoma: A Case Illustration
- Corresponding Author:
- C Zachariadi
Department of Neurology, KAT General Hospital of Attika, Nikis, Greece
E-mail: [email protected]
Received date: 24-Jan-2023, Manuscript No. NPY-23-91545; Editor assigned: 26-Jan-2023, PreQC No. NPY-23-91545 (PQ); Reviewed Date: 09-Feb-2023, QC No NPY-23-91545; Revised date: 16-Feb-2023, Manuscript No. NPY-23-91545 (R); Published date: 24-Feb-2023, DOI:10.37532/1758-2008.2023.13(1).642
Abstract
Lymphoma of the Central Nervous System (CNSL) represents a rare subset of non-Hodgkin lymphoma, divided in two subgroups: Primary and secondary CNS lymphoma. The latter refers to systemic non-Hodgkin lymphoma that has disseminated to the CNS.
We present the case of a 73-year-old-male with progressively deteriorating weakness in his left upper extremity over the past month, accompanied by gait disorder with frequent falls. The patient was diagnosed with non-Hodgkin Lymphoma two years prior to his assessment. One and a half years later he was treated with combined immunochemotherapy due to extensive relapse, leading to partial remission. We performed a CSF flow cytometry, which identified monoclonal B-cells at 45% of the CD45+ cells. The Advanced MRI Spine and Brain Protocol performed, including Diffusion, Perfusion and MR-Spectroscopy methods, revealed enhancing lesions of the cervical spinal cord and the brain, after the i.v. administration of gadolinium. It also revealed intense diffusion restriction, typical perfusion histogram for a lymphoma combined with an almost normal spectroscopy, indicative of secondary lymphoma of the CNS. We immediately proceeded to treatment with high dose intravenous corticosteroid, which led to partial remission and the patient was soon able to walk more steadily.
According to our literature search, there is only one other published case that reports Hodgkin’s lymphoma affecting the intramedullary spinal cord and no other case with simultaneously insult of the brain and intramedullary spinal cord.
Keywords
Central nervous system lymphoma, intramedullary spinal cord lymphoma, spinal cord tumor, spinal cord metastasis, MR-Spectroscopy, advanced imaging modalities
Abbreviations
NHL: Non-Hodgkin’s Lymphoma; CNS: Central Nervous System; CNSL: Central Nervous System Lymphoma; CSF: Cerebrospinal Fluid; MR-Spectroscopy: Magnetic Resonance Spectroscopy; DLBCL: Diffuse Large B-Cell Lymphoma; R-CHOP: Rituximab-Cyclophosphamide Hydroxydaunorubicin Oncovin Prednisone; FTP: Fractional Tumor Burden; rCBV: relative Cerebral Blood Volume
Introduction
Lymphoma of the Central Nervous System (CNSL) represents a rare subset of non-Hodgkin lymphoma. According to the World’s Health Organization definition, CNSL is divided in two subgroups: Primary central nervous system lymphoma refers to those cases confined to the CNS parenchyma, meninges, cranial nerves, spinal cord and/or eye. On the other hand, Secondary CNS Lymphoma (SCNSL) refers to systemic non-Hodgkin lymphoma that has disseminated to the CNS [1,2]. This subgroup is also referred to as metastatic lymphoma. According to literature data, approximately 70% of SCNSL cases present with leptomeningeal spread while one-third presents with brain parenchymal disease, affecting mostly the frontal lobes and the basal ganglia [3]. Reports in the literature of secondary intramedullary spinal cord NHL are rare and confined to case reports [4,5].
SCNSL is an infrequent complication of systemic lymphoma. This frequency depends at least partially upon the aggressiveness of the NHL subtype, and it varies from 2%-10% among patients with aggressive subtypes of systemic NHL (e.g. Diffuse Large B Cell Lymphoma (DLBCL)) [6-8]. The incidence appears to be lower in indolent NHL, while prophylaxis plays additionally a protective role. According to literature data SCNSL may occur in up to 50% of cases of Burkitt/lymphoblastic lymphoma or AIDS-related lymphoma when no prophylaxis is given [9]. SCNSL has been proposed to arise from hematogenous arterial spread, direct invasion, venous spread via Batson’s plexus and perilymphatic spread [10,11].
The diagnosis of SCNSL usually requires a combination of clinical presentation, radiological manifestations (enhanced MRI) and cerebral spinal fluid tests conventional cytology and flow cytometry [12-14].
Case Presentation
A 73-year-old-male presented at the emergency room of our tertiary hospital acclaiming progressively deteriorating weakness in his left upper extremity over the past month, accompanied by gait disorder with frequent falls. The weakness had worsened the week prior to his presentation at our clinic. He did not complain of neck pain. According to his medical history, the patient was diagnosed with Non-Hodgkin Lymphoma (NHL) in October 2017. The lymphoma was revealed as an incidental finding in a chest X-ray and the diagnosis was established with FNA biopsy of the chest wall mass. At the time of the diagnosis the disease was staged as grade IVa. Considering the aggravated past medical history of the patient (coronary heart disease, chronic obstructive pulmonary disease, and pulmonary embolism) as well as the low grade of the lymphoma, the treating physicians decided not to initiate chemotherapy, instead to closely monitor the patient. The disease remained stable for two years. The PET/CT scan performed for follow-up in September 2020 showed an extensive thoracic relapse. The patient was then treated with combined immunochemotherapy (R-CHOP) from December 2020 until April 2021. The following PET/CT scan in May 2021 showed partial remission of the disease.
The patient was admitted from the ER to our clinic in August 2021. At that time the physical examination revealed severely decreased muscle strength of 1/5 in the left upper extremity distal and a T5 sensory level. The reflexes were brisk in the left upper extremity, whereas the plantar response was extensor bilaterally. The patient demonstrated a broad-based gate. No bladder dysfunction was detected.
▪ Work up
Laboratory tests were within normal range except a mild elevation of the white blood cell count. Inflammatory conditions were ruled out with negative blood titers.
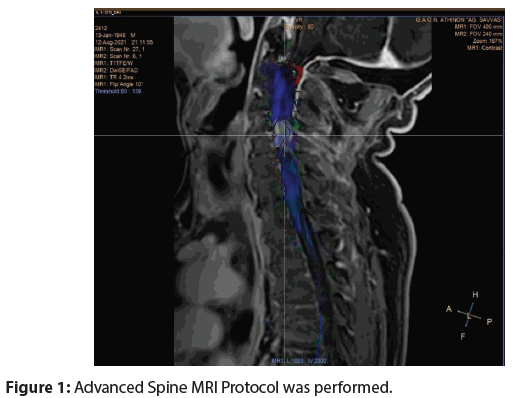
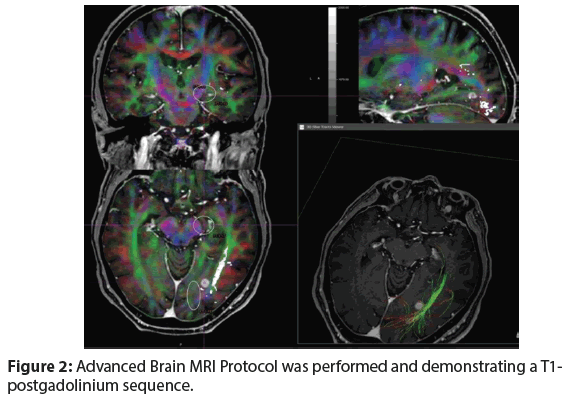
A lumbar puncture revealed protein of 26 mg/dL and white cell count 2/μL with 18% lymphocytes. CSF cytology was negative, whereas flow cytometry identified monoclοnal B-cells at 45% of the CD45+ cells (CD19+CD22+CD79b+CD5-CD23- CD43+kappa-lamda+). An Advanced Spine and Brain MRI Protocol including Diffusion, Perfusion, MR-Spectroscopy, as well as Diffusion Tensor Imaging-Fiber Tractography (DTI-FT) of the spine and brain was performed (Figures 1 and 2).
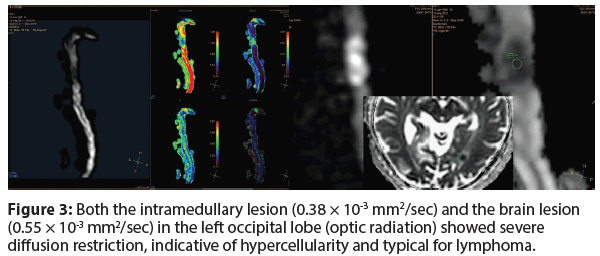
A severe diffusion restriction with high signal intensity on b value=1000 s/mm2 and low signal/values on ADC map was revealed in the intramedullary lesion (0.38 × 10-3 mm2/sec), as well as in the brain lesion (0.55 × 10-3 mm2/sec) in the left occipital lobe (optic radiation), indicative of hypercellularity and typical for lymphoma (Figure 3).
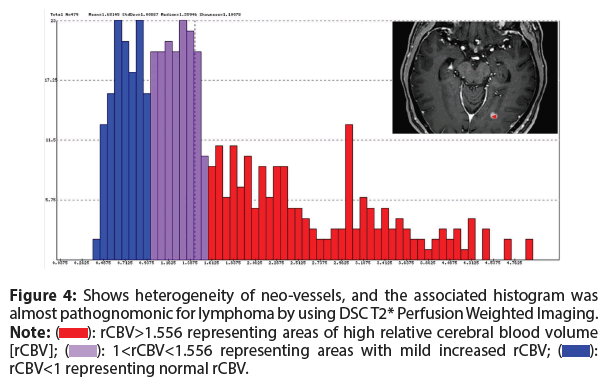
The DSC T2* Perfusion Weighted Imaging in the strongly, almost homogeneously, enhancing intracranial lesion showed a heterogeneity of neo-vessels with the corresponding histogram almost pathognomonic for lymphoma (peak values of blue and purple to the right side of the graphic, while red on the left side) (Figure 4).
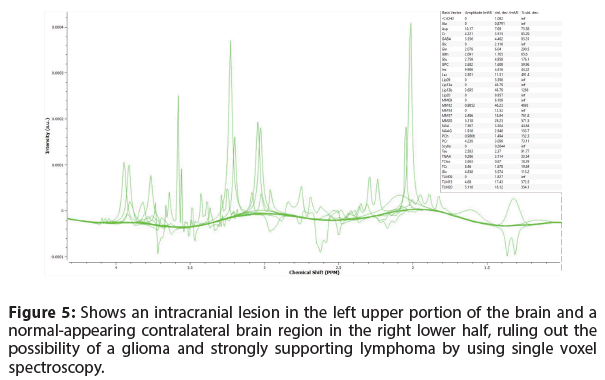
Furthermore, the Single Voxel Spectroscopy demonstrated intermediate TE=144 ms of the intracranial lesion (left upper part), as well as normal appearing corresponding contralateral brain region (right lower part), a feature which excludes the possibility of a glioma and is strongly indicative for lymphoma (Figure 5).
A stereotactic biopsy of the lesions was ineffective. He subsequently underwent an opthalmological as well as urological evaluation that revealed the absence of any intraocular or testicular involvement.
Figure 4: Shows heterogeneity of neo-vessels, and the associated histogram was almost pathognomonic for lymphoma by using DSC T2* Perfusion Weighted Imaging.
Note: (): rCBV>1.556 representing areas of high relative cerebral blood volume [rCBV]; (): 1<rCBV<1.556 representing areas with mild increased rCBV; (): rCBV<1 representing normal rCBV.
▪ Differential diagnosis
Our initial differential diagnosis based on the MRI findings included broadly malignant versus infectious, inflammatory etiology. Malignant considerations included relapsed NHL with CNS involvement, primary CNS malignancy or metastatic disease from a different primary oncological process. Infectious etiologies included opportunistic infections, HIV, multiple abscesses, especially considering that the patient had received a combined immunochemotherapy previously. Inflammatory processes with or without demyelination, paraneoplastic myelopathy, were also included in the spectrum of our differential diagnosis. The investigation process listed above and especially the remarkable findings of the MRI and MR-Spectroscopy in combination with the CNS flow cytometry helped us effectively rule out other processes and confirm our initial hypothesis of a secondary CNS Lymphoma with brain and cervical spine involvement. Furthermore, we consider the lesion of the cervical cord responsible for the patient’s semiology, whereas the brain lesions seem to appear asymptomatic.
▪ Treatment
We immediately initiated a high dose intravenous corticosteroid therapy with 1 gr Methylprednisolone/d for five consecutive days. The patient showed signs of remission after the first day of therapy and by the end of it were able to walk more steadily, while he had regained function in his left upper extremity.
Our patient was then referred to his treating hematooncologist and combination chemotherapy with Ibrutinib 500 mg/d and high dose methotrexate 3.5 mg (every two weeks, up to eight circles) was planned for initiation in the following weeks.
Results and Discussion
SCNSL is defined as lymphoma involvement both within and outside of the CNS at the time of diagnosis or as the CNS relapse of a systemic lymphoma [15]. CNS relapse is reported to mainly occur within the first year after diagnosis (median, 6 months) [16]. NHL of the CNS may affect the brain parenchyma, leptomeninges, spinal cord, spinal nerve roots, or epidural space. In the literature, primary or secondary NHL in the brain and meninges are commonly encountered, in contrast with secondary involvement of the NHL in the intramedullary spinal cord, which remains a rare entity. The spinal cord is more commonly affected by an epidural primary or secondary deposit of NHL within the spinal canal [17]. On the other hand, reports of systemic Hodgkin’s lymphoma affecting the CNS are rare, with prevalence between 0.2% and 0.5% [18]. Overall the incidence of CNS relapse seems to have decreased after the introduction of rituximab following a change in the pattern of CNS relapse, with a predominance of parenchymal over leptomeningeal relapse [1,15,19].
In our report we present a patient with secondary brain as well as intramedullary Lymphoma in the context of a relapse. To our knowledge, there is only one other case in literature that reports Hodgkin’s lymphoma affecting the intramedullary spinal cord and no other case with simultaneously insult of the brain and intramedullary spinal cord [20]. T-cell lymphoma also appears to rarely affect the spinal cord given the number of cases reported in literature [21,22].
Regarding the presenting symptoms of intramedullary Lymphoma, weakness, sensory loss, spasticity, pain, and bowel or bladder incontinence appear to be common. In our case, the patient presented with symptoms indicative of a cervical myelopathy. Severely decreased muscle strength in the left upper extremity, as well as T5 sensory level with brisk reflexes and extensor plantar responses were the core neurological symptoms at the time of admission. Considering the past medical history of the patient, our suspicion for lymphoma was high. On the other hand, the patient was immunocompromised after having received therapy for the NHL in the recent past, so that an inflammatory disease or primary spinal cord tumor like ependymoma remained a possible differential diagnosis.
The investigation of choice in the modality of imaging for extranodal lymphoma with CNS semiology is the MRI of the brain and spine. Lesion biopsy and CSF cytology as well as flow cytometry are instrumental in establishing the diagnosis of spinal cord lymphoma [12,20,23,24]. However, in most of the studies reviewed, the findings were not based on a combination of MRI examinations and histological findings. In several studies, the diagnosis was made by CSF examination, by CT, or only from clinical data without histological or radiological validation [1,6,25-27]. For example, in a study published by Villa, et al. only 55% of their patients underwent MRI or CT9; the diagnosis was made using cerebrospinal fluid flow cytometry or only from clinical data, without histological or imaging confirmation.
The MRI findings of our patient were further investigated with an Advanced-MRI Protocol, including Diffusion, DSC-T2* perfusion and MR-Spectroscopy showing almost pathognomonic findings for lymphoma. In combination with the results of the CSF flow cytometry the diagnosis of secondary CNS lymphoma was established with intracerebral as well as intramedullary involvement. It should be noted that steroid treatment could create artefacts in the CSF cytology and thus, a sample of CSF must always be collected prior to steroid administration. In our case though, we initiated immediately a steroid treatment, and collected the CSF sample a couple of weeks later. The delay was due to technical inability of our laboratory to conduct a CSF flow cytometry at that moment.
We did not proceed to a lesion biopsy in our patient. The role of biopsy of the spinal cord in diagnosing a lymphoma is not clear. The safety of a spinal cord biopsy remains uncertain in the literature, regarding the high risk of severe irreversible spinal cord damage [28]. The role of biopsies should be limited to patients with non-conclusive imaging and laboratory findings.
The diagnosis of CNS lymphoma on CSF cytology/flow cytometry and imaging modalities requires immediate initiation of treatment. Because of the low incidence of lymphomas of the spinal cord, there is a lack of established standards of management. The majority of the patients appear to respond with full remission to high dose intravenous chemotherapy [29-31]. The most used systemic therapy is immunomodulators like methotrexate, alkylating agents, or monoclonal antibodies. Our patient was referred to his treating hematooncologist and combination chemotherapy with Ibrutinib 500 mg/d and high dose methotrexate 3.5 mg (every two weeks, up to eight circles) was initiated [32-34]. The treatment plan was organized according to the underlying comorbidity, age and recent immunochemotherapy of the patient. According to our literature search Rituximab has also been used in limited case studies with good outcome and tolerance [35,36].
The role of radiotherapy and surgery remains secondary and may be used as palliative therapy in patients who cannot tolerate high dose chemotherapy or escalation therapy in patients who did not reach full remission with chemotherapy. The results are often poor and the side effects include toxicity of the brain and spinal cord with severe neurological damage [23,37,38].
Conclusion
In our paper a rare case of a SCNSL with involvement of the brain as well as the cervical spinal cord is reported. With the help of CSF flow cytometry and advanced imaging modalities (with enhanced and metabolic methods) we were able to establish the diagnosis and promptly initiate a treatment schedule. High dose corticosteroids, ibrutinib and methotrexate were included in our patient’s therapy plan. Our patient showed a good response to the initial treatment with intravenous steroids, and we await the initiation of the chemo- and immunotherapy to evaluate his further clinical and radiological response. At this point, we would like to emphasize the fact that, significant symptom improvement and tumor size reduction in the radiological follow-up should always raise suspicion for CNS lymphoma. Aggressive treatment with combined systemic chemotherapy remains the golden standard, while the overall estimated survival rate is estimated around 32%.
Unfortunately, our patient deceased from chemotherapy complications (lympopenia, sepsis) before we could perform a new MRI Scan.
References
- Malikova H, Burghardtova M, Koubska E, et al. Secondary central nervous system lymphoma: spectrum of morphological MRI appearances. Neuropsychiatr. Dis. Treat 14, 733-740 (2018).
[Crossref] [Google Scholar] [PubMed]
- Jahnke K, Thiel E, Martus P, et al. Retrospective study of prognostic factors in non-Hodgkin lymphoma secondarily involving the central nervous system. Ann. Hematol 85(1), 45-50 (2006).
[Crossref] [Google Scholar] [PubMed]
- Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: Characteristic findings on traditional and advanced imaging. AJNR. Am. J. Neuroradiol 32(6), 984-992 (2011).
[Crossref] [Google Scholar] [PubMed]
- Mathur S, Law AJ, Hung N. Late intramedullary spinal cord metastasis in a patient with lymphoblastic lymphoma: Case report. J. Clin. Neurosci 7(3), 264-268 (2000).
[Crossref] [Google Scholar] [PubMed]
- Wong CME, van Heesewijk JP, Ramos LM. Intramedullary non-Hodgkin’s lymphoma of the spinal cord; A case report. Eur. J. Radiol 12(3), 226-227 (1991).
[Crossref] [Google Scholar] [PubMed]
- Hollender A, Kvaloy S, Nome O, et al. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: A risk model. Ann. Oncol 13(7), 1099-1107 (2002).
[Crossref] [Google Scholar] [PubMed]
- Nishikori M, Sorachi K, Doi S, et al. Central nervous system involvement in non-Hodgkin’s lymphoma. Eur J. Cancer. Clin. Oncol 27(11), 2051-2058 (1986).
[Google Scholar] [PubMed]
- Bernstein SH, Unger JM, Leblanc M, et al. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: A 20-year follow-up analysis of SWOG 851-the Southwest Oncology Group. J. Clin. Oncol 27(1), 114-119 (2009).
[Crossref] [Google Scholar] [PubMed]
- Villa D, Connors JM, Shenkier TN, et al. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: The impact of the addition of rituximab to CHOP chemotherapy. Ann. Oncol 21(5), 1046-1052 (2010).
- Costigan DA, Winkelman MD. Intramedullary spinal cord metastasis. A clinicopathological study of 13 cases. J. Neurosurg 62(2), 227-233 (1985).
[Crossref] [Google Scholar] [PubMed]
- Batson OV. The function of the vertebral veins and their role in the spread of metastases. Clin. Orthop 112(1), 138-49 (1940).
[Crossref] [Google Scholar] [PubMed]
- Feng L, Chen D, Zhou H, et al. Spinal primary central nervous system lymphoma: Case report and literature review. J. Clin. Neurosci 50, 16-19 (2018).
[Crossref] [Google Scholar] [PubMed]
- Jiménez MDM, Vicente LG, Alonso RC, et al. The multiple faces of nervous system lymphoma. atypical magnetic resonance imaging features and contribution of the advanced imaging. Curr. Probl. Diagn. Radiol 46(2), 136-145 (2017).
- Mapelli P, Vanoli EG, Picchio M, et al. Spinal cord involvement secondary to non-Hodgkin’s lymphoma identified by 18F-FDG PET/CT. Rev. Esp. Med. Nucl. E. Imagen. Mol 32(2), 125-125 (2013).
[Crossref] [Google Scholar] [PubMed]
- Maciocia P, Badat M, Cheesman S, et al. Treatment of diffuse large B-cell lymphoma with secondary central nervous system involvement: encouraging efficacy using CNS-penetrating R-IDARAM chemotherapy. Br. J. Haematol 172(4), 545-553 (2016).
[Crossref] [Google Scholar] [PubMed]
- Qualls D, Abramson JS. Advances in risk assessment and prophylaxis for central nervous system relapse in diffuse large B-cell lymphoma. Haematologica 104(1), 25-34 (2019).
[Crossref] [Google Scholar] [PubMed]
- Roug IK, McCartney LB. Metastatic non-Hodgkin lymphoma presenting as low back pain and radiculopathy: A case report. J. Chiropr. Med 11(3), 202-206 (2012).
[Crossref] [Google Scholar] [PubMed]
- Galán L, Sánchez AC, Cantos B, et al. Central nervous system involvement in Hodgkin’s lymphoma. Med. Oncol 28(Suppl 1), S505-508 (2011).
[Crossref] [Google Scholar] [PubMed]
- Siegal T, Goldschmidt N. CNS prophylaxis in diffuse large B-cell lymphoma: If, when, how and for whom? Blood. Rev 26(3), 97-106 (2012).
[Crossref] [Google Scholar] [PubMed]
- D’Cruz J, Adeeb N, Von Burton G, et al. Diagnosis and management of intramedullary spinal cord lymphoma: A case illustration and review of literature. Interdiscip. Neurosurg 19: 100552 (2020).
- Guzzetta M, Drexler S, Buonocore B, et al. Primary CNS T-cell lymphoma of the spinal cord: case report and literature review. Lab. Med 46(2), 159-163 (2015).
[Crossref] [Google Scholar] [PubMed]
- Morita M, Osawa M, Naruse H, et al. Primary NK/T-cell lymphoma of the cauda equina: a case report and literature review. Spine 34(24), E882-885 (2009).
[Crossref] [Google Scholar] [PubMed]
- Rao A, Griffiths R, Arnaoutakis K. Paralyzed by a rare cause: an unusual case of metastatic diffuse large B cell lymphoma of the intramedullary spinal cord. Ann. Hematol 93(2), 337-338 (2014).
[Crossref] [Google Scholar] [PubMed]
- Benz R, Viecelli A, Taverna C, et al. Paroxysmal non-kinesigenic dyskinesia due to spinal cord infiltration of low-grade B cell non-Hodgkin’s lymphoma. Ann. Hematol 91(3), 463-465 (2012).
[Crossref] [Google Scholar] [PubMed]
- Zinzani P, Magagnoli M, Frezza G, et al. Isolated central nervous system relapse in aggressive non-Hodgkin’s lymphoma: The Bologna experience. Leuk. Lymphoma 32(5-6), 571-576 (1999). [Crossref]
[Google Scholar] [PubMed]
- Keldsen N, Michalski W, Bentzen SM, et al. Risk factors for central nervous system involvement in Non-Hodgkins-Lymphoma a multivariate analysis. Acta. Oncol 35(6), 703-708 (1996).
[Crossref] [Google Scholar] [PubMed]
- Van Besien K, Ha CS, Murphy S, et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood 91(4), 1178-1184 (1998).
[Crossref] [Google Scholar] [PubMed]
- Cohen-Gadol AA, Zikel OM, Miller GM, et al. Spinal cord biopsy: A review of 38 cases. Neurosurgery 52(4), 806-815 discussion 815-816 (2003).
[Crossref] [Google Scholar] [PubMed]
- Broen M, Draak T, Riedl RG, et al. Diffuse large B-cell lymphoma of the cauda equina. BMJ Case Rep 2014, bcr2014205950 (2014).
[Crossref] [Google Scholar] [PubMed]
- Guzik G. Primary b-cell spinal cord lymphoma of the cervical spine. Ortop. Traumatol. Rehabil 20(3), 219-227 (2018).
[Crossref] [Google Scholar] [PubMed]
- Ezon IC, Barteselli G, Rosenberg J, et al. Concurrent primary vitreoretinal and spinal cord lymphoma: A unique entity. JAMA Ophthalmol 132(7), 902 (2014).
[Crossref] [Google Scholar] [PubMed]
- Lv L, Sun X, Wu Y, et al. Efficacy and safety of ibrutinib in central nervous system lymphoma: A PRISMA-Compliant single-arm meta-analysis. Front. Oncol 11, 2523 (2021).
[Crossref] [Google Scholar] [PubMed]
- Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II “proof-of-concept” iLOC study by the Lymphoma study association (LYSA) and the French Oculo-Cerebral lymphoma (LOC) network. Eur. J. Cancer 117: 121-130 (2019).
[Crossref] [Google Scholar] [PubMed]
- T Low J, B Peters K. Ibrutinib in primary central nervous system diffuse large B-cell lymphoma. CNS Oncol 9(1), CNS51.
[Crossref] [Google Scholar] [PubMed]
- Steffanoni S, Doorduijin JK. Narrative review: Secondary central nervous system lymphoma. Ann Lymphoma 5(0) (2021).
- Doorduijn JK, van Imhoff GW, van der Holt B, et al. Treatment of secondary central nervous system lymphoma with intrathecal rituximab, high-dose methotrexate, and R-DHAP followed by autologous stem cell transplantation: Results of the HOVON 80 phase 2 study. Hematol. Oncol 35(4), 497-503 (2017).
[Crossref] [Google Scholar] [PubMed]
- Hanssens PE, Lagerwaard FJ, Levendag PC. Principles of radiotherapy of neoplastic meningosis. J. Neurooncol 38(2-3), 145-150 (1998).
[Crossref] [Google Scholar] [PubMed]
- Liu W, Yang Y, Qi S, et al. Treatment, survival, and prognosis of advanced-stage natural killer/t-cell lymphoma: An analysis from the china lymphoma collaborative group. Front. Oncol 10, 583050 (2021).
[Crossref] [Google Scholar] [PubMed]