Research Article - Neuropsychiatry (2016) Volume 6, Issue 5
Effects of social stress on ethanol responsivity in adult mice
- Corresponding Author:
- Dr Maria A Aguilar
Departamento de Psicobiología, Facultad de Psicología, Universitat de Valencia, Avda. Blasco Ibáñez, 2146010 Valencia, Spain
Tel: 34-96 386 46 11
Fax: 34-96 386 46 68
Abstract
Exposure to stressors can produce behavioural and neurochemical adaptations that render individuals more prone to drug-seeking and drug-taking behaviours. Alcohol is the most consumed drug of abuse in the USA and Europe and binge drinking is becoming increasingly frequent. Stress experiences are a risk factor for alcohol abuse in humans and recent studies in animal models reported that repeated social stress increased alcohol consumption. The aim of the present work is to evaluate the acute or long-term effects of stress induced by social defeat (SD) on consumption of ethanol (ETOH 6%) using the two-bottle choice procedure and on place conditioning induced by this substance.
Keywords
Ethanol, Social defeat, Place conditioning, Two-bottle choice, Mice
Introduction
Many people take drugs in the world for different reasons, but drugs of abuse are powerfully reinforcing and may cause intense subjective effects that, once experienced, lead to further experimentation and drug taking experiences [1].These substances are hypothesized to highjack the function of neural circuits that encode motivational and rewarding signals [2]. In fact, drug addiction is considered a multifactorial disorder and drug dependence has become one of the main concerns worldwide with associated personal, social and health problems [3].
Although alcohol consumption with social drinking pattern is acceptable in our societies, this drug is the most commonly used addictive drug worldwide, and its use place a major socioeconomic and public health burden on modern societies. In Spain, alcohol is the main legal drug substance consumed [4] and the World Health Organization (WHO) estimates that approximately 2, 5 million people die every year from ethanol use. Nowadays, alcoholism is recognized as a true “toxic pandemy” and it is considered the addictive disorder with the highest expansion rate among all population groups [5].
It is well known that different environmental factors such as stressful situations can alter drug consumption in humans and experimental animals [6-8]. An important aspect to take into consideration in the animal models used to study the influence of the social environment in the development of drug abuse is the motivation of animal for get or takes the addictive substance. Environmental stimuli that are associated with consumption, or explicitly signal, the availability of alcohol can powerfully evoke alcohol seeking and consummator behaviours [9]. The place conditioning paradigm allows evaluating the conditioned motivational effects of alcohol and the role of environmental stimuli to evoke drug seeking. However, this paradigm has the inconvenience that the animal does not choose the consumption of the drug, which is a problematic issue when the effects of a drug with aversive properties are evaluated. Conversely, in the self-administration or the two-bottle choice procedure the voluntary consumption of ETOH can be studied (Figures 1 and 2).
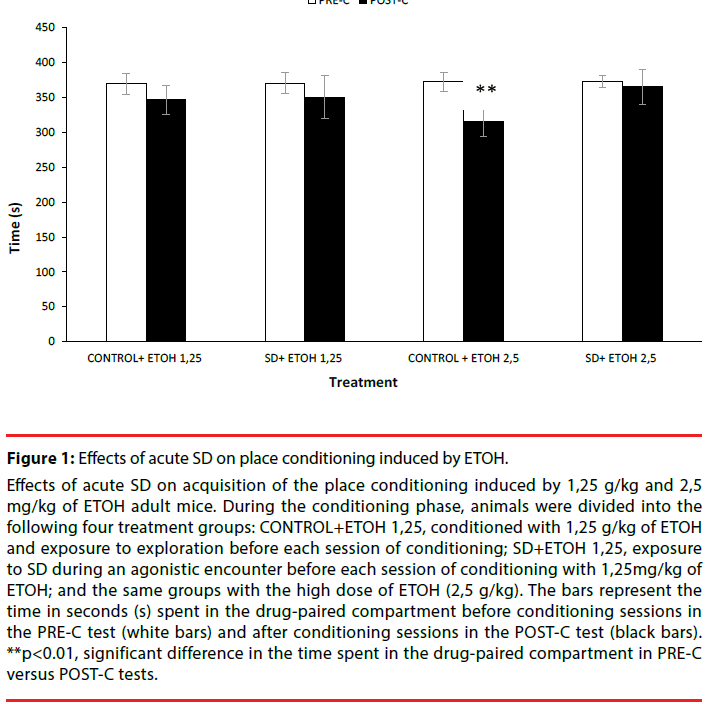
Effects of acute SD on acquisition of the place conditioning induced by 1,25 g/kg and 2,5 mg/kg of ETOH adult mice. During the conditioning phase, animals were divided into the following four treatment groups: CONTROL+ETOH 1,25, conditioned with 1,25 g/kg of ETOH and exposure to exploration before each session of conditioning; SD+ETOH 1,25, exposure to SD during an agonistic encounter before each session of conditioning with 1,25mg/kg of ETOH; and the same groups with the high dose of ETOH (2,5 g/kg). The bars represent the time in seconds (s) spent in the drug-paired compartment before conditioning sessions in the PRE-C test (white bars) and after conditioning sessions in the POST-C test (black bars). **p<0.01, significant difference in the time spent in the drug-paired compartment in PRE-C versus POST-C tests.
Figure 1: Effects of acute SD on place conditioning induced by ETOH.
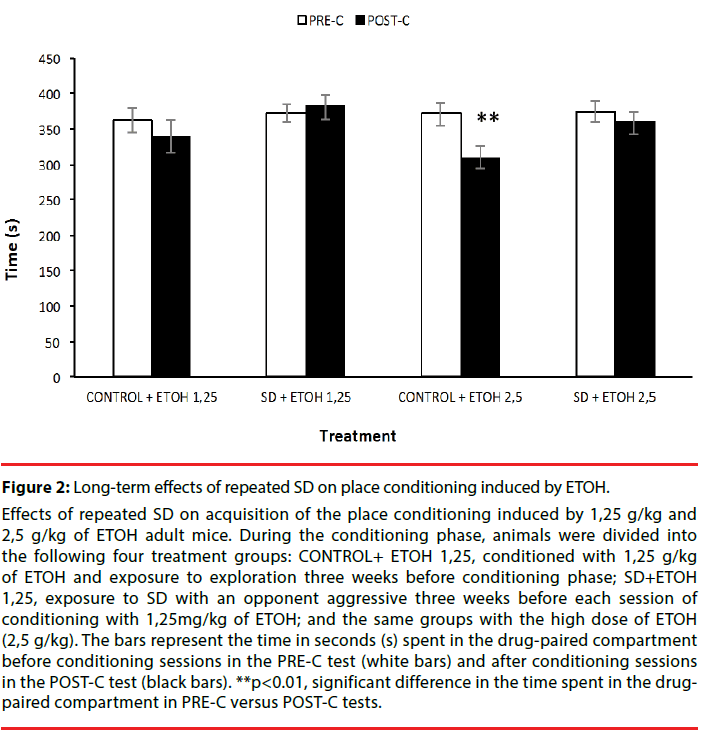
Effects of repeated SD on acquisition of the place conditioning induced by 1,25 g/kg and 2,5 g/kg of ETOH adult mice. During the conditioning phase, animals were divided into the following four treatment groups: CONTROL+ ETOH 1,25, conditioned with 1,25 g/kg of ETOH and exposure to exploration three weeks before conditioning phase; SD+ETOH 1,25, exposure to SD with an opponent aggressive three weeks before each session of conditioning with 1,25mg/kg of ETOH; and the same groups with the high dose of ETOH (2,5 g/kg). The bars represent the time in seconds (s) spent in the drug-paired compartment before conditioning sessions in the PRE-C test (white bars) and after conditioning sessions in the POST-C test (black bars). **p<0.01, significant difference in the time spent in the drugpaired compartment in PRE-C versus POST-C tests.
Figure 2: Long-term effects of repeated SD on place conditioning induced by ETOH.
Different studies have shown that to exposure to different procedures of social defeat (SD) plays a major role in the initiation and escalation of psychostimulant consumption, as well as in reinstatement of drug seeking after extinction, in the place conditioning and self-administration paradigms [8,10-14]. There are different forms to induce SD in the animals, according if the social stress is acute vs repeated, or using an agonistic encounter in neutral environment vs the resident/intruder procedure. Moreover,the effects of SD can also vary in function of the time elapsed between stress exposure and drug administration. In particular, the effects of SD stress on alcohol consumption change as a function of the temporal parameters of stress exposure in relation with alcohol access. Different protocols of repeated SD increased ethanol self-administration in mice and rats none previously exposed to this drug [15- 18]. Conversely, SD reduces alcohol intake in rodents that had previously acquired selfadministration or when is administered immediately before access to the drug [18-20]. In the place conditioning paradigm, it has been observed that exposure to SD immediately prior to conditioning sessions with ethanol reduces the conditioned place aversion (CPA) induced by this drug in rats [20], while exposure to repeated SD for 19 days increased the conditioned place preference (CPP) induced by this substance in mice [21], delayed its extinction and exacerbated reinstatement [22]. Moreover, this kind of social stress increased ethanol intake in the two-bottle choice procedure [21].
The aim of the present study is to evaluate if social stress exposure modifies the motivational effects of ETOH in the place conditioning paradigm and the intake of this drug in the two-bottle choice procedure and if there are differences in the effects of the stress depending on the voluntary or involuntary administration of ETOH. In particular, we aim to determine the effects of acute SD exposure before place conditioning or ETOH access and the longterm effects of repeated social stress situation on both measures to evaluate if there are differences between two types of social stress.
Methods
▪ Subjects
Male OF1 mice (n=195) of 42 days of age were acquired to Charles River (Barcelona, Spain). We have used this strain of mice because in previous studies we observed that exposure to SD modify the vulnerability of mice to different drugs of abuse including alcohol [17]. All mice (except those used as aggressive opponents) were housed in groups of four in plastic cages (25×25×14.5 cm) for 8 days before the experiments began. To reduce their stress levels in response to experimental manipulations, mice were handled for 5 min/day on each of the 3 days before initiation of the behavioural procedures. Adult mice used as aggressive opponents (n=15) were individually housed in plastic cages (23×13.5×13 cm) for a month before experiments to induce heightened aggression [23]. All mice were housed under the following conditions: constant temperature; a reversed light schedule (white lights on 19:30–07:30 h); and food and water freely available, except during behavioural tests. Procedures involving mice and their care were carried out in compliance with national, regional and local laws and regulations, which are in compliance with the Directive 2010/63/EU.
▪ Apparatus
For place conditioning, we used eight identical Plexiglas boxes with two equally sized compartments (30.7 cm long×31.5 cm wide×34.5 cm high) separated by a grey central area (13.8 cm long×31.5 cm wide×34.5 cm high). The compartments had different-coloured walls (black vs. white) and distinct floor textures (fine grid in the black compartment and wide grid in the white one). Four infrared light beams in each compartment of the box and six in the central area allowed the recording of the position of the animals and their crossings from one compartment to the other. The equipment was controlled by three IBM PC computers using Monpre 2Z software (Cibertec, SA, Madrid, Spain).
▪ Drugs
Absolute ETOH of 96% purity (Scharlab SL, Barcelona, Spain) in different dose (1,25 g/kg and 2,5 g/kg) was diluted in physiological saline (0.9% NaCl) and administered i.p. with a volume of 0.02 ml/g before place conditioning session. In the two-bottle choice paradigm, animals were presented to intermittent concurrent access (on alternating days) to 6% ETOH (v/v) and water in their home-cage. Oral voluntary consumption was assessed every 24h.
▪ Procedure of SD
To induce SD stress, the animals underwent two different types of SD exposure.
Acute agonistic encounters in a neutral area: To induce acute SD stress (ASD), the animals were subjected to a 10-min agonistic encounter with an adult aggressive opponent in a neutral transparent plastic cage (23×13.5×13 cm). Mice in the ASD groups showed avoidance/ flee and defensive/submissive behaviours after suffering aggression (threat and attack) from an opponent, as observed in previous studies [23- 26]. The criterion used to define an animal as defeated was the adoption of a specific posture signifying defeat, characterized by an upright submissive position, limp forepaws, upwardly angled head and retracted ears [24,27]. All agonistic encounters were videotaped to confirm the presence of SD. Control animals [NASD groups] did not suffer SD, but instead explored the neutral transparent plastic cages for 10 min without contact with an opponent. These encounters were performed immediately before place conditioning or access to ETOH.
Repeated SD using the resident/intruder paradigm: In this paradigm, which is more common, a territorial resident mouse confronts and dominates an intruder, which is the experimental animal [8]. In each episode of repeated SD (RSD), the “intruder” is introduced into the home cage of an experienced aggressive male resident, where it is threatened and attacked by the resident until it shows clear signs of submission, usually after a few minutes of confrontation. Each brief episode of SD consists of three phases. During 10 minutes in the initial phase, the intruder is placed inside the resident’s cage separated by a barrier that protects the intruder from attacks by the resident but allows social contact and species-typical threats from the male aggressive resident. In the second phase during 5 minutes, the protective barrier is removed allowing the confrontation. A defeat is defined when the intruder displays a supine posture for five consecutive seconds, a response that typically occurs after few biting attacks from the resident. In the third and final phase, the protective barrier is placed for another 10 min to allow social threats from the resident. Socially defeat-stressed animal are exposed to four episodes of SD separated by intervals of 2 days (for example on days 1, 4, 7 and 10) [27,28]. All agonistic encounters were videotaped to confirm the presence of SD. Control groups (NRSD) was introduced into the cage without an opponent. The long-term effects of this type of stress on the motivational effects of ETOH and intake of this drug were evaluated three weeks after the last SD.
▪ Place conditioning procedure
This procedure was carried out during the dark cycle following a procedure that was unbiased in terms of initial spontaneous preference [29,30]. The acquisition of place conditioning consists in three phases: Pre-conditioning (PRE-C), conditioning and Post-conditioning (POST-C).
During PRE-C, the time spent by the animal in each compartment during a 15-min period was recorded. Eight animals showing a strong unconditioned aversion or a preference for any compartment were excluded from the study. In the second phase (conditioning), experimental animals were conditioned with ETOH immediately before being confined to the drugpaired compartment for 30 min on days 4, 5, 6, 7, 8, 9 and after six hours the animals received saline before being confined to the vehicle-paired compartment for 30 min on the same days. During the third, POST-C phase, the time spent by the untreated mice in each compartment was recorded during a 15-min period.
▪ Voluntary ethanol drinking: Two-bottle choice procedure
The two-bottle choice drinking procedure was carried out as previously described, with minor modifications [21,31,32]. Mice receive three 24-h sessions of free access to two-bottle choice (water and 6% ETOH) per week (typically Monday, Wednesday, and Friday), with 24-h and 48-h withdrawal periods during weekdays and weekends, respectively. During the withdrawal periods, mice receive two bottles of water. The placement of the ETOH bottle is alternated each drinking session to control for side preferences [33,34]. The volume of ETOH and water consumed were measured every day. Throughout the experiment, evaporation/spillage estimates were calculated daily from 2 pipettes placed in an empty cage, one containing drinking water and the other containing the appropriate ETOH solution.
Experimental design
Experiment 1: Effect of acute social stress on the acquisition of place conditioning induced by ethanol.
To evaluate the effect of SD during an agonistic encounter on the rewarding effects of two different doses of ethanol, four groups of animals were used. Two groups of adult mice were conditioned with 1,25 g/kg of ETOH on postnatal day (PND) 56, 57, 58, 59, 60 and 61. One of these was subjected to a SD stress before each session of conditioning with ETOH (SD+ETOH 1,25) whereas another group did not undergo stress, but remained for 10 min in the neutral area (performing only exploration) immediately before each session of conditioning with ETOH (Control+ETOH 1,25). The same protocol was applied to the other two groups of animals but conditioned with ETOH 2,5 g/kg (SD+ETOH 2,5; Control+ETOH 2,5).
Experiment 2: Effect of repeated social stress on the acquisition of place conditioning induced by ethanol.
To evaluate the long-term effects of repeated social stress on the motivational effects of different doses of ethanol four groups of animals were used. Two groups of adult mice were conditioned with 1,25 g/kg of ETOH on PND 88, 89, 90, 91, 92 and 93. One of these groups was exposed to intermittent SD stress following the resident-intruder paradigm three weeks before conditioning with ETOH (SD+ETOH 1,25), whereas another group did not undergo stress, but remained in the cage without opponent (performing exploration) during the same times that stressed animal (Control+ETOH 1,25). The same protocol was applied to the other two groups of animals but conditioned with ETOH 2,5 g/kg (SD+ETOH 2,5; Control+ETOH 2,5).
Experiment 3: Effect of acute social stress on ETOH intake in the two-bottle choice procedure.
To evaluate the effect of SD during an agonistic encounter on the rewarding effects of ETOH, in a voluntary pattern of consumption, two groups of adult mice were used. One group suffered SD stress during 10 min on PND 53,55,57,62 and immediately were housed in its home cage with free access to 2-bottle choice (water and 6% ethanol) during 24 h. Afterwards, the animals received two-bottles of water in a withdrawal period of 24 h (SD+ETOH 6%). Another group did not undergo stress, but remained for 10 min in the neutral area (for exploration) immediately before access to ETOH (6%) in its home cage (Control+ETOH 6%).
Experiment 4: Effect of repeated social stress on ETOH intake in the two-bottle choice procedure.
To evaluate the long-term effects of repeated SD on the ETOH intake in a voluntary pattern of consumption two groups of adult mice were used. One group suffered SD stress in the intruderresident paradigm on PND 53,55,57,62 and after 3 weeks mice have free access to 2-bottle choice (water and ETOH 6%) during 24 h in its home cage. Afterwards, the animals received two-bottles of water in a withdrawal period of 24h (SD+ETOH 6%). Another group did not undergo stress, but remained in the empty cage without opponent (for exploration) immediately before access to ETOH (6%) in its home cage (Control+ETOH 6%) (Figures 3 and 4).
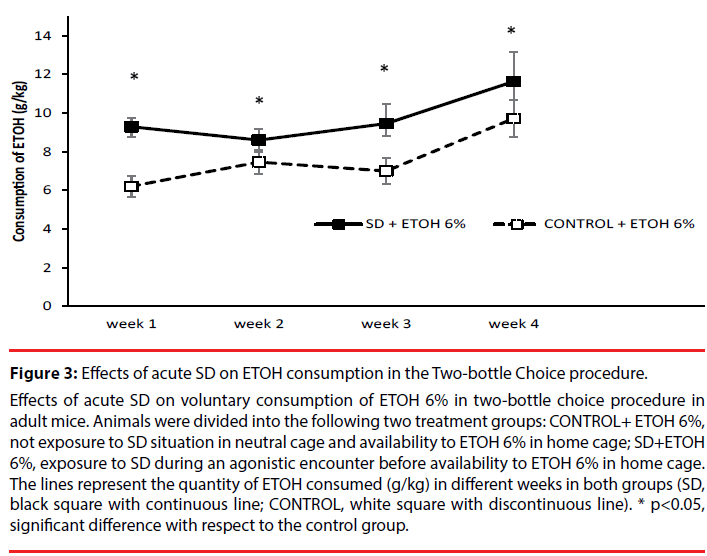
Effects of acute SD on voluntary consumption of ETOH 6% in two-bottle choice procedure in adult mice. Animals were divided into the following two treatment groups: CONTROL+ ETOH 6%, not exposure to SD situation in neutral cage and availability to ETOH 6% in home cage; SD+ETOH 6%, exposure to SD during an agonistic encounter before availability to ETOH 6% in home cage. The lines represent the quantity of ETOH consumed (g/kg) in different weeks in both groups (SD, black square with continuous line; CONTROL, white square with discontinuous line). * p<0.05, significant difference with respect to the control group.
Figure 3: Effects of acute SD on ETOH consumption in the Two-bottle Choice procedure.
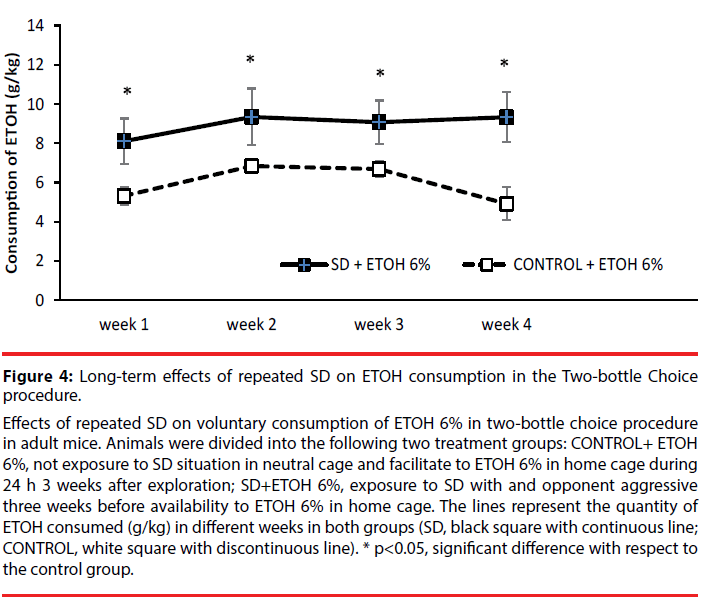
Effects of repeated SD on voluntary consumption of ETOH 6% in two-bottle choice procedure in adult mice. Animals were divided into the following two treatment groups: CONTROL+ ETOH 6%, not exposure to SD situation in neutral cage and facilitate to ETOH 6% in home cage during 24 h 3 weeks after exploration; SD+ETOH 6%, exposure to SD with and opponent aggressive three weeks before availability to ETOH 6% in home cage. The lines represent the quantity of ETOH consumed (g/kg) in different weeks in both groups (SD, black square with continuous line; CONTROL, white square with discontinuous line). * p<0.05, significant difference with respect to the control group.
Figure 4: Long-term effects of repeated SD on ETOH consumption in the Two-bottle Choice procedure.
▪ Statistical Analysis
To analyse data of the acute SD or repeated social stress in ETOH place conditioning, twoway ANOVAs with a within-subjects variable Days with two levels (PRE-C and POST-C) and two between-subjects variables Stress with two levels (CONTROL and SD) and Dose with two levels (1,25 and 2,5 g/kg) were used. To analyse data of both types of SD on two-bottle choice procedure, two-way ANOVAs with a withinsubjects variable Weeks with four levels (week 1-4) and one between subjects variable Stress with two levels (CONTROL and SD) was used. All post-hoc comparisons were performed with Bonferroni tests.
Results
Experiment 1: Effect of acute social stress on the acquisition of place conditioning induced by ethanol.
The ANOVA showed that the variable Days [F(1,49)=5.644; p<0.05] was significant. Posthoc comparison showed that the control (nonstressed) group treated with the high dose of ETOH (Control+2,5 g/kg) significantly spent less time in ETOH-paired compartment in POST-C than in PRE-C, suggesting that the high dose of ETOH induces place aversion (p<0.01).
Experiment 2: Effect of repeated social stress on the acquisition of place conditioning induced by ethanol.
The ANOVA showed that the variable Days [F(1,47)=4.540; p<0.05] was significant. Posthoc comparison showed that the control (nonstressed) group treated with the high dose of ETOH (Control+2,5 g/kg) significantly spent less time in ETOH-paired compartment in POST-C than in PRE-C, suggesting that the high dose of ETOH induces place aversion (p<0.01).
Experiment 3: Effect of acute social stress on ETOH intake in the two-bottle choice procedure.
The ANOVA showed that the variables Weeks [F(3,75)=9.604; p<0.001] and Stress [F(1, 25)=5.506; p<0.05] were significant. Post-hoc comparisons showed that in the fourth week mice drink more ETOH than in the other weeks (p<0.05) and stressed mice drink more than controls (p<0.05).
Experiment 4: Effect of repeated social stress on ETOH intake in the two-bottle choice procedure.
The ANOVA showed that the variables Weeks [F(3,81)=3.71; p<0.05] and Stress [F(1,27)=7.21; p<0.01] were significant. Post-hoc comparisons showed that mice drink more ETOH in week 2 than in week 1 (p<0.05). Moreover, stressed animals drink more that controls (p<0.05).
Discussion
The present study demonstrated that exposure to SD modifies the ability of alcohol to induced motivational effects in the place conditioning paradigm and its consumption in the two-bottle choice procedure. In particular, both acute and repeated SD reduced the place aversion induced by the high dose of ethanol (that is only observed in control but not in defeated mice) and increased the voluntary consumption of this drug.
With respect to the results of place conditioning, we observed that mice conditioned with the high dose of ethanol (2,5 g/kg) showed CPA to the compartment associated with the drug. A previous study of our laboratory with mice of the same strain to those used in the present work demonstrated that it is not possible to induce CPP in young adult male mice of this strain with different doses of ethanol (0,625, 1,25 or 2,5 g/kg), while using the same procedure of place conditioning a clear CPP is observed in females of the same age and in male and female adolescent mice [30]. CPP have been classically used to evaluate the motivational effects of drugs because pairs a distinct environment with the pharmacological effect of a drug [35]. Typically, animals prefer the environment that is paired with drugs of abuse [36], but there is an important exception with ethanol, in which CPA is the common outcome in rats [37-39]. This CPA is not related with a lack of reinforcing effects of ethanol since rats effectively self-administered this drug [40,41], although a negative correlation has been observed between conditioned taste aversion and ethanol drinking, suggesting that the aversive actions of this substance may limit its consumption [42]. Moreover, the positive rewarding and negative aversive effects of drugs are independent processes [43].
Using CPP, normally mice show a preference for ethanol-paired environments and cues [44-54] for example, moderate doses of ethanol (1.5-2 g/ kg) produced a significant CPP in C57BL/6 mice [55-57]. However, other studies showed CPA [38,58,59] and by modifying procedural details like the schedule of ethanol administration both CPP and CPA can be observed with the same dose of ethanol [58-62]. A recent study performed with the same procedure of place conditioning that we used in the present work, but with a different strain of mice, reported that systemic administration of 1.5 g/kg of ethanol induced robust CPP [63]. Since, it has been demonstrated that induction of CPP by ethanol depends on the strain of animals in question [64], we believe that the CPA observed in the present study is mainly due to the strain of mice used. Modifying different procedural details (as the number and duration of conditioning sessions), we never had obtained place conditioning with adult male mice of this strain (unpublished findings; [30]. With the procedure used in the present study we observed that OF1 adult mice showed CPA with the high dose of alcohol (2,5 g/kg) while lower doses (1,25 g/kg) did not induce motivational effects. These results confirm the idea that the effect of ethanol in the place conditioning paradigm depends on genetic variables and that OF1 mice are more sensible to the negative effects of ethanol.
With respect to the results of the two-bottle choice procedure we observed that mice drink alcohol, although only a slight increase in the intake of this substance through the time of the experiment is observed. The lack of a clear progressive increase in ethanol drinking can be related with the aversive effects of ethanol in this strain of mice, although a recent study of our laboratory has demonstrated that they effectively acquire operant ethanol self-administration [17].
The most important result found in this study is that social stress has important effects in the motivational properties of alcohol and in its consumption because stress exposure reversed the negative effects of alcohol in the place conditioning paradigm and increased its intake in the two-bottle choice procedure. It is important to note that the reduction of CPA induced by ethanol and the increased intake is consistently observed both after acute and longterm exposure to SD stress. According with our results, a previous study demonstrated that SD exposure immediately prior to conditioning sessions with ethanol reduces the place aversion induced by this drug in rats [20]. Moreover, it has been reported that repeated SD increased ethanol self-administration in mice and rats [15,16,18] and that chronic social stress increased ethanol intake in the two-bottle choice procedure in mice [21]. The novelty of the present study is the demonstration that SD stress induces longterm changes on the motivational properties and intake of alcohol, since the inhibition of ethanol CPA and the increased intake of this drug can be observed three weeks after stress exposure. In accordance with these long-term effects of SD, we have recently demonstrated that repeated intermittent SD during adolescence induces a long-term increase in operant self-administration of ethanol [17].
The reduction in the ethanol CPA induced by the exposure to SD stress can be related with the high intake of this substance. It has been suggested that drug taking may be a function of the relative balance between drug reward and aversion [43], and it has been demonstrated that ethanol’s aversive actions limited its oral consumption [42,43]. We can hypothesise that the stressed animals do not suffer the negative effects of alcohol in the same intensity than nonstressed animals, and consequently they increase its consumption.
In conclusion, the results presented in this study show that SD experiences induced a short- and long-term increase in the ethanol drinking. It could be interesting in future studies to measure the levels of different neurotransmitters such as dopamine, glutamate or corticotrophin-releasing factor in mice exposed to defeat and ethanol and to correlate biochemical and behavioural changes to explore the role of these neurotransmitter systems in the enhancing effects of SD stress on the vulnerability to alcohol consumption.
Acknowledgements
The present work has been possible thanks to the following grants: Ministerio de Economía y Competitividad (MINECO), Dirección General de Investigación, PSI2014-51847, Instituto de Salud Carlos III, Red de Trastornos Adictivos (RTA) RD12/0028/0005 and Unión Europea, Fondos FEDER “una manera de hacer Europa”. Generalitat Valenciana, Conselleria de Educación, PROMETEOII/2014/063.
References
- Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories–indications for novel treatments of addiction. Eur. J.Neurosci40(1), 2163-2182 (2014).
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol24(2), 97-129 (2001).
- HosseinzadehMichalak A, BiaÃâ¦Ãâa G. Alcohol dependence-Neurobiology and treatment. Acta. Pol. Pharm73(1), 3-12 (2016).
- Encuestadomiciliariasobre alcohol y drogas en España (EDADES) 2012/2013. Delegación del gobiernopara el plan nacionalsobredrogas. Ministerio de sanidad, política social e igualdad. (2013).
- Szalontay AS. Physiopathological and therapeutical correlations in alcohol dependence. Rev. Med.Chir. Soc. Med. Nat. Iasi 118(3), 692-698 (2014).
- Goeders NE. Stress and cocaine addiction. J.Pharmacol. Exp.Ther301(3), 785-789 (2002).
- Sinha R, Fox HC, Hong KIA, et al.Effects of adrenal sensitivity, stress-and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch. Gen. Psychiatry 68(9), 942-952 (2011).
- Miczek KA, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol.Ther120(2), 102-128 (2008).
- Field M, Wiers RW, Christiansen P, et al.Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol. Clin. Exp. Res34(8), 1346-52 (2010).
- Aguilar MA, Garcia-Pardo MP, Montagud-Romero S, et al.Impact of social stress in addition to psychostimulants: what we know from animal models. Curr. Pharm. Des19(40), 7009-7025 (2013).
- García-Pardo MP, Rodríguez-Arias M, Maldonado C, et al.Effects of acute social stress on the conditioned place preference induced by MDMA in adolescent and adult mice. Behav.Pharmacol25(5 and 6), 532-546 (2014).
- Montagud-Romero S, Aguilar MA, Maldonado C, et al.Acute social defeat stress increases the conditioned rewarding effects of cocaine in adult but not in adolescent mice. Pharmacol. Biochem. Behav135(1), 1-12 (2015).
- Rodríguez-Arias M, García-Pardo MP, Montagud-Romero S, et al.The role of stress in psychostimulant addiction: treatment approaches based on animal models. Drug use and abuse, Nova Science Publishers Inc. New York, USA, 153-220 (2013).
- RodríguezâÃâ¬ÃÂArias M, MontagudâÃâ¬ÃÂRomero S, RubioâÃâ¬ÃÂAraiz A, et al.Effects of repeated social defeat on adolescent mice on cocaineâÃâ¬ÃÂinduced CPP and selfâÃâ¬ÃÂadministration in adulthood: integrity of the blood-brain barrier. Addict.Biol(2015).
- Caldwell EE, Riccio DC. Alcohol self-administration in rats: modulation by temporal parameters related to repeated mild social defeat stress. Alcohol 44(3), 265-274 (2010).
- Riga D, Schmitz LJ, van der Harst JE, et al. A sustained depressive state promotes a guanfacine reversible susceptibility to alcohol seeking in rats. Neuropsychopharmacology 39(5), 1115-1124 (2014).
- RodriguezâÃâ¬ÃÂArias M, Navarrete F, BlancoâÃâ¬ÃÂGandia MC, et al.Social defeat in adolescent mice increases vulnerability to alcohol consumption. Addict.Biol(2016).
- Norman KJ, Seiden JA, Klickstein JA, et al.Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology 232(6), 991-1001 (2015).
- Van Erp AMM, Tachi N, Miczek KA. Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav.Pharmacol12(5), 335-342 (2001).
- Funk D, Vohra S, Le AD. Influence of stressors on the rewarding effects of alcohol in Wistar rats: studies with alcohol deprivation and place conditioning. Psychopharmacology 176(1), 82-87 (2004).
- Bahi A. Increased anxiety, voluntary alcohol consumption and ethanol-induced place preference in mice following chronic psychosocial stress. Stress 16(4), 441-451 (2013).
- Bahi A, Dreyer JL. Chronic psychosocial stress causes delayed extinction and exacerbates reinstatement of ethanol-induced conditioned place preference in mice. Psychopharmacology 231(2), 367-377 (2014).
- Rodṟ̉guez-Arias M, Minarro J, Aguilar MA, et al.Effects of risperidone and SCH 23390 on isolation-induced aggression in male mice. Eur. Neuropsychopharmacol8(2), 95-103 (1998).
- Do Couto BR, Aguilar MA, Manzanedo C, et al. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology185(4), 459-470 (2006).
- Do Couto BR, Aguilar MA, Lluch J, et al. Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology 207(3), 485-498 (2009).
- Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science 215(4539), 1520-1522 (1982).
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol.Behav53(5), 983-993 (1993).
- Quadros IM, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology 206(1), 109-120 (2009).
- Manzanedo C, Aguilar MA, RodrÃâñÃÅÃÂguez-Arias M, et al.Effects of dopamine antagonists with different receptor blockade profiles on morphine-induced place preference in male mice. Behav. Brain. Res121(1), 189-197 (2001).
- Roger-Sánchez C, Aguilar MA, Rodríguez-Arias M, et al.Age-and sex-related differences in the acquisition and reinstatement of ethanol CPP in mice. Neurotoxicol.Teratol34(1), 108-115 (2012).
- BahiA. The selective metabotropic glutamate receptor 7 allosteric agonist AMN082 prevents reinstatement of extinguished ethanol-induced conditioned place preference in mice. Pharmacol.Biochem.Behav101(2), 193-200 (2012).
- Giuliano C, Goodlett CR, Economidou D, et al.The Novel μ-Opioid Receptor Antagonist GSK1521498 Decreases Both Alcohol Seeking and Drinking: Evidence from a New Preclinical Model of Alcohol Seeking. Neuropsychopharmacology 40(13), 2981-2992 (2015).
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line–derived neurotrophic factor. Alcohol43(1), 35-43 (2009).
- Barak S, Carnicella S, Yowell QV,et al.Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J.Neurosci31(27), 9885-9894 (2011).
- Aguilar MA, Rodríguez-Arias M, Miñarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain. Res. Rev59(2), 253-277 (2009).
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol12(3-4), 227-462 (2007).
- Gauvin DV, Briscoe RJ, Goulden KL, et al. Aversive attributes of ethanol can be attenuated by dyadic social interaction in the rat. Alcohol11(3), 247-251 (1994).
- Stewart RB, Murphy JM, McBride WJ, et al.Place conditioning with alcohol in alcohol-preferring and-nonpreferring rats. Pharmacol.Biochem.Behav53(3), 487-491 (1996).
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat.Protoc1(4), 1662-1670 (2006).
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int. Rev.Neurobiol107-145 (2003).
- Camarini R, Marcos Pautassi R, Mendez M, et al.Behavioral and neurochemical studies in distinct animal models of ethanol's motivational effects. Curr. Drug. Abuse. Rev3(4), 205-221 (2010).
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol 42(1), 1-11 (2008).
- Verendeev A, Riley AL. The role of the aversive effects of drugs in self-administration: assessing the balance of reward and aversion in drug-taking behavior. Behav.Pharmacol24(5 and 6), 363-374 (2013).
- Cunningham CL, Patel P. Rapid induction of Pavlovian approach to an ethanol-paired visual cue in mice. Psychopharmacology 192(2), 231-241 (2007).
- Song M, Wang XY, Zhao M, et al.Role of Stress in Acquisition of AlcoholâÃâ¬ÃÂConditioned Place Preference in Adolescent and Adult Mice. Alcohol. Clin. Exp. Res31(12), 2001-2005 (2007).
- Houchi H, Warnault V, Barbier E, et al. Involvement of A2A receptors in anxiolytic, locomotor and motivational properties of ethanol in mice. Genes. Brain. Behav7(8), 887-898 (2008).
- Newton PM, Zeng L, Wang V, et al.A blocker of N-and T-type voltage-gated calcium channels attenuates ethanol-induced intoxication, place preference, self-administration, and reinstatement. J.Neurosci28(45), 11712-11719 (2008).
- Font L, Miquel M, Aragon CM. Involvement of brain catalase activity in the acquisition of ethanol-induced conditioned place preference. Physiol.Behav93(4), 733-741 (2008).
- Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology 191(2), 195-202 (2007).
- Gremel CM, Cunningham CL. Roles of the nucleus accumbens and amygdala in the acquisition and expression of ethanol-conditioned behavior in mice. J.Neurosci28(5), 1076-1084 (2008).
- Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: empirical and theoretical analysis. Psychopharmacology 201(1), 97-106 (2008).
- Groblewski PA, Lattal KM, Cunningham CL. Effects of dâÃâ¬ÃÂCycloserine on Extinction and Reconditioning of EthanolâÃâ¬ÃÂSeeking Behavior in Mice. Alcohol. Clin. Exp. Res33(5), 772-782 (2009).
- Itzhak Y, Roger-Sánchez C, Anderson KL. Role of the nNOS gene in ethanol-induced conditioned place preference in mice. Alcohol 43(4), 285-291 (2009).
- Herlhag E, Egecioglu E, Landgren S, et al.Requirement of central ghrelin signaling for alcohol reward. Proc. Natl. Acad. Sci. U S A106(27), 11318-11323 (2009).
- Gamsby JJ, Templeton EL, Bonvini LA, et al.The circadian Per1 and Per2 genes influence alcohol intake, reinforcement, and blood alcohol levels. Behav. Brain. Res249, 15-21 (2013).
- Griffin III WC, McGovern RW, Bell GH, et al.Interactive effects of methylphenidate and alcohol on discrimination, conditioned place preference and motor coordination in C57BL/6J mice. Psychopharmacology 225(3), 613-625 (2013).
- Hilbert ML, May CE, Griffin WC. Conditioned reinforcement and locomotor activating effects of caffeine and ethanol combinations in mice. Pharmacol.Biochem.Behav110(1), 168-173 (2013).
- Bechtholt AJ, Gremel CM, Cunningham CL. Handling blocks expression of conditioned place aversion but not conditioned place preference produced by ethanol in mice. Pharmacol. Biochem. Behav79(4), 739-744 (2004).
- Font L, Aragon CM, Miquel M. Ethanol-induced conditioned place preference, but not aversion, is blocked by treatment with D-penicillamine, an inactivation agent for acetaldehyde. Psychopharmacology 184(1), 56-64 (2006).
- Cunningham CL, Henderson CM, Bormann NM. Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology 139(1-2), 62-70 (1998).
- Cunningham CL, Henderson CM. Ethanol-induced conditioned place aversion in mice. Behav.Pharmacol11(7-8), 591-602 (2000).
- Cunningham CL, Clemans JM, Fidler TL. Injection timing determines whether intragastric ethanol produces conditioned place preference or aversion in mice. Pharmacol.Biochem.Behav72(3), 659-668 (2002).
- Al Maamari E, Al Ameri M, Al Mansouri S, et al.Inhibition of urokinase plasminogen activator “uPA” activity alters ethanol consumption and conditioned place preference in mice. Drug. Des.Devel.Ther8(1), 1391 (2014).
- Cunningham CL. Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behav.Neurosci128(4), 430 (2014).