Research Article - Neuropsychiatry (2018) Volume 8, Issue 1
Do Benzodiazepines Plus Fluvoxamine Cause a Rapid Increase in Serum Brain-derived Neurotrophic Factor or Clinical Improvement in Major Depressive Disorder Patients?
- *Corresponding Author:
- Reiji Yoshimura, M.D., Ph.D
Department of Psychiatry, University of Occupational and Environmental Health
1-1 Yahatanishi-ku, Kitakyushu 8078555, Japan
(Tel) +81936917253
(Fax) +81936924894
Abstract
Abstract
Objective: Benzodiazepines are sometimes co-prescribed with antidepressants for patients with major depressive disorder (MDD) who have symptoms such as sleep disturbance, restlessness, or anxiety. The aim of the present study was to examine whether serum levels of brain-derived neurotrophic factor (BDNF) differed between patients with MDD treated with fluvoxamine alone and those treated with a fluvoxamine and benzodiazepine combination.
Methods: Twenty-eight first-episode patients with MDD were enrolled in the present study. Thirteen patients were male, and 15 were female, with ages ranging from 29 to 70 (mean ± standard deviation [SD], 47.9 ± 11.6) years. All the patients met the DSM-5 criteria for MDD; were physically healthy; and were free of alcohol or drug abuse, comorbid anxiety, or personality disorders at the time of the study. The patients were administered fluvoxamine monotherapy for eight weeks at doses that varied among the patients and were not fixed for ethical reasons. In the benzodiazepine co-administration group, the dose of benzodiazepines was kept constant during the experimental period. Depression was assessed using the Structured Interview Guide for the 17-item Hamilton Depression Rating Scale (HAMD17). Serum BDNF and plasma fluvoxamine levels were measured.
Results: The HAMD17 scores in all subjects were significantly decreased after treatment with fluvoxamine. No significant difference was found between the two groups (fluvoxamine versus fluvoxamine and benzodiazepines) regarding the reduction in HAMD17 scores. Serum BDNF levels in all subjects were significantly increased after treatment with fluvoxamine. No difference was observed between the groups regarding the increase in serum BDNF levels.
Conclusion: These results suggest that benzodiazepine co-administration did not influence serum BDNF levels or clinical improvement in MDD patients.
Keywords
Benzodiazepine, Brain-derived neurotrophic factor, Major depressive disorder, Fluvoxamine
Introduction
Major depressive disorder (MDD) is a common and major psychiatric disorder that affects as many as 20% of individuals within their lifetime [1-3]. A wide variety of pharmacological treatments are available for treating depression, including tricyclic antidepressants, monoamine oxidase inhibitors, and selective serotonin reuptake inhibitors (SSRIs). Fluvoxamine, an SSRI that is widely used for treating depression and other psychiatric disorders, has been suggested to have early effects when used as an antidepressant drug [4,5]. Additionally, the results of a meta-analysis have shown that significant improvements in Hamilton Rating Scale for Depression (HAMD) scores that were achieved in the first few weeks were maintained after 6 weeks of treatment [6]. Sleep disturbances and anxiety are common co-occurring disturbances in patients with MDD and deserve particular attention. Benzodiazepines are sometimes co-prescribed with antidepressants for patients with such symptoms. However, there is no convincing evidence to show that such a combination is more effective than antidepressants alone, and there is no clear indication for using this combination. In addition, clinicians must be cautious when prescribing benzodiazepines due to the potential for patients to become dependent. To date, benzodiazepines are still prescribed along with antidepressant drugs for MDD patients in clinical practice.
Brain-derived neurotrophic factor (BDNF) plays important roles in several physiological functions of neurons, which might be related to the pathophysiology of psychiatric disorders, such as mood disorders and schizophrenia [7-9]. Several meta-analyses have demonstrated that serum or plasma BDNF levels are abnormally low in patients with MDD and that BDNF levels are elevated following a course of antidepressant treatment. Although the pathophysiology of depression and the mechanisms of action of antidepressant drugs have yet to be determined, measurement of blood BDNF levels may have potential use as a biomarker for MDD or as a predictor of antidepressant efficacy [10,11] because peripheral BDNF partially originates from the brain [12].
This study aimed to examine whether serum levels of BDNF differ between patients with MDD treated with fluvoxamine alone and patients treated with a fluvoxamine and benzodiazepine combination. If the combination of benzodiazepines with fluvoxamine accelerates the increase in BDNF levels, this therapeutic strategy might be reasonable because it would bring rapid relief of depressive symptoms, reinforcing the evidence for co-prescribing benzodiazepines in a short targeting period for insomnia and/or anxiety. The effects of benzodiazepine on BDNF expression, however, remain controversial. Several reports have found decreased [13], increased [14,15] or unchanged levels of BDNF and/or c-fos. To the best of our knowledge, no studies have compared BDNF levels between patients taking antidepressants with benzodiazepines and those taking antidepressants alone. Therefore, to examine this question, we compared serum BDNF levels between MDD patients who took fluvoxamine alone and those who took fluvoxamine plus benzodiazepines.
Materials and Methods
▪ Participants
Twenty-eight patients with MDD were enrolled in the present study. Thirteen patients were male, and 15 were female. Twenty-eight patients with MDD were enrolled in the present study. Thirteen patients were male, and 15 were female, with ages ranging from 29 to 70 (mean ± standard deviation [SD], 47.9 ± 11.6) years. Enrolled criteria were as follows, (1) met the DSM-5 criteria for MDD, (2) first-episode of MDD, (3) physically healthy, (4) fluvoxamine monotherapy. Exclusion criteria were also as follows, (1) alcohol or drug abuse, (2) comorbid anxiety, or personality disorders. Prior to the study, all subjects provided written informed consent after receiving a full explanation of the study and of any potential risks and benefits of study participation. The study was approved by the Ethics Committee of the University of Occupational Environmental Health and was conducted according to the Declaration of Helsinki II. The demographics of the participants are shown in Table 1. Their ages ranged from 29 to 70 (mean ± standard deviation [SD] 47.9 ± 11.6) years. All patients met the MDD criteria of DSM-5, were physically healthy and were free of alcohol or drug abuse, comorbid anxiety, or personality disorders at the time of the study. All patients consented to participate after being informed of the study’s purpose. The patients received fluvoxamine monotherapy for eight weeks at doses that varied and were not fixed for ethical reasons. In the benzodiazepine co-administration group, the dose of benzodiazepines was kept constant during the experimental period.
▪ Assessment of clinical variables
Depression was assessed by an experienced psychiatrist (R.Y.) using the Structured Interview Guide for the 17-item Hamilton Depression Rating Scale (HAMD17).
▪ Serum BDNF assay
Blood was drawn at 9:00 AM. Serum levels of BDNF were measured in duplicate using a human BDNF ELISA Kit (Adipo Bioscience, Santa Clara, CA, USA). All experiments were performed in duplicate according to the manufacturer’s instructions. The optical density of each well was measured using an automated microplate reader (Emax; Molecular Devices, Sunnyvale, CA, USA).
▪ Plasma fluvoxamine assay
The plasma fluvoxamine level was measured using high-performance liquid chromatography according to a method that we previously described [17]. All experiments were performed in duplicate.
Statistical analysis
Student’s t-test was used to compare the two groups. Multilevel regression model was used in which repeated measurements were nested in individuals. The model used BDNF and HAM-D as a dependent variable and group and time point as independent variables. All analyses were performed using Stata 14.0 software (Stata Statistical Software: College Station, TX, USA).
Results
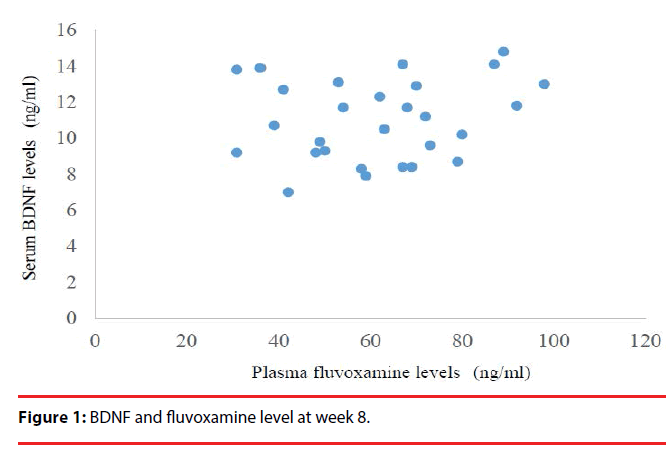
The demographic data for each group are shown in Table 1, and the benzodiazepine data are shown in Table 2. The mean values and standard deviations (SDs) for all response variables in each treatment group at each measurement time point are shown in Table 3. The mean values and standard errors for the primary outcome measure, BDNF levels, are shown in Table 3. Serum BDNF levels in all subjects were significantly increased at week 4 (p=0.016) and at week 8 (p<0.001) after treatment with fluvoxamine (Table 3). No difference in the increase in serum BDNF levels was observed between the groups (fluvoxamine versus fluvoxamine and benzodiazepines) (p=0.299). The HAMD17 scores of all subjects were significantly decreased at weeks 4 (p<0.001) and 8 (p<0.001) after treatment with fluvoxamine (Table 3). There were no significant differences between the two groups in the reduction in HAMD17 scores (p=0.809), plasma fluvoxamine levels or serum BDNF levels (Figure 1). A significant correlation was found between the serum BDNF levels and the HAMD in all subjects (p<0.001) (Figure 2).
| fluvoxamine | fluvoxamine and benzodiazepines | p-value | |
|---|---|---|---|
| N | 14 | 14 | |
| Age | 45.6±11.6 | 50.3±11.3 | .307 |
| Gender male/female | 6/8 | 7/7 | .705 |
| Dose of fluvoxamine at 8 weeks | 116.1±27.7 | 123.2±25.8 | .502 |
| Dose of benzodiazepine at 8 weeks (diazepam eqv. mg) | 4.6±2.0 | ||
| Fluvoxamine levels at 8 weeks (ng/ml) | 61.7±19.3 | 61.6±16.9 | .992 |
Table 1: Demographics of each group.
| Benzodiazepines | |
|---|---|
| Alprazolam | 3 |
| Triazolam | 2 |
| Lorazepam | 3 |
| Brotizolam | 3 |
| Flunitrazepam | 3 |
Table 2: Benzodiazepines.
| time | mean | SD | mean | SD | p | mean | SD | Mean | SD | p |
|---|---|---|---|---|---|---|---|---|---|---|
| baseline | 9.71 | 2.32 | 9.54 | 1.89 | 21.86 | 2.71 | 22.14 | 2.53 | ||
| 4weeks | 10.29 | 2.12 | 10.07 | 1.81 | 0.016 | 16.71 | 2.95 | 16.82 | 2.80 | <0.001 |
| 8weeks | 10.67 | 2.54 | 11.35 | 1.90 | <0.001 | 11.71 | 3.73 | 11.64 | 3.69 | <0.001 |
Table 3: Changes of BDNF and HAM-D.
Discussion
The major finding of the present study was that co-administration of benzodiazepine and fluvoxamine did not accelerate serum BDNF increase or the additional clinical improvement of depressive symptoms. A recent meta-analysis demonstrated that blood (serum or plasma) levels of mature BDNF in patients with MDD were lower than those in healthy controls. Lowered blood BDNF levels increased over the course of antidepressant treatment [18-20].
The serum BDNF level reflects the severity of depressive state, because a significant correlation was found between the serum BDNF and the HAMD scores. In short, serum BDNF is a state biomarker for major depressive disorder. Thus, we can know the efficacy of antidepressants or/ and benzodiazepines from the changing the serum BDNF level. Acute administration of both triazolam and zolpidem resulted in reductions in BDNF protein and c-fos protein expression in the hippocampus [13]. Acute, but not chronic, administration of zolpidem specifically reduced exon IV-containing BDNF transcripts with concomitant increases in the association of methyl CpG-binding protein 2 with the BDNF gene promoter IV region and the association of the phosphor-cAMP response element-binding protein with BDNF gene promoter I region [13]. Several other researchers have reported that diazepam-induced c-fos expression increased or did not change in rodent brain tissue or cell lines [21]. Panford-Walsh, et al. [22] reported a significant increase in BDNF expression after salicylate was completely reversed through the application of midazolam. Sarabi, et al. [23] reported that psychoactive drugs, including benzodiazepines, significantly increased serum BDNF levels, which was associated with better survival in depressed patients with colorectal cancer in a clinical setting.
Zafra, et al. [24] reported GABAergic regulation is associated with BDNF expression in rats. The authors insisted that subtle changes in the balance between the glutamatergic and the GABAergic systems significantly alter expression of BDNF and NGF in the hippocampus and influence the amount of NGF protein available for the septal cholinergic neurons. Guilloux, et al. [25] reported both direct (low RNA/ protein) and indirect (low BDNF-dependent gene pattern) evidence for reduced BDNF function in the amygdala of female subjects with MDD in postmortem study. The authors also demonstrated by using mutant mice models that a complex mechanism of low constitutive and activity-dependent BDNF function in MDD, particularly affecting somatostatin / neuropeptide Y-related GABA neurons, thus linking the neurotrophic and GABA hypotheses of depression.In contrast, neither acute nor chronic treatment with chlordiazepoxide have been reported to regulate BDNF protein levels in the brain [26]. Reported that baclofen, a GABAB receptor agonist exerts a direct effect on serum levels of BDNF in alcohol-dependent patients [27]. Benzodiazepines do not primarily affect biogenic amine uptake or metabolism, although they do augment gamma-amino butyric acid (GABA) activity. The antidepressant efficacy of benzodiazepines, which are GABAA receptor agonists, is consistent with the GABA theory of depression [28]. Actually, benzodiazepine may have more rapid action in some patients. If so, fluvoxamine with benzodiazepine rapidly increases serum BDNF better than without benzodiazepine. The results in the present study did not support the hypothesis.
Taken together, the results did not seem consistent. To date, it remains unknown whether treatment with benzodiazepines alone alters serum BDNF. Antidepressants, including fluvoxamine, significantly increase serum BDNF, which is considered to partially explain their antidepressant efficacy. To the best of our knowledge, few studies have investigated the effects of benzodiazepine on serum BDNF in MDD. Therefore, the results of the present study do not contradict the general understanding that serum BDNF reflects a depressive state and that benzodiazepine and fluvoxamine produce similar outcomes. The present study had several limitations, including i) the absence of a healthy control group, ii) the absence of fluvoxamine plus placebo group, iii) a small sample size, and iv) the lack of a randomized controlled trial design. Thus, further rigorous investigations must be conducted to confirm these preliminary findings. In conclusion, benzodiazepine coadministration did not influence serum BDNF levels or clinical improvement in MDD patients.
Conflict of interest
Dr. Katsuki has received speaker’s honoraria from Dainippon Sumitomo, Meiji.
Dr. Fjino declares no conflicts of interest associated with this manuscript.
Dr. Yoshimura has received speaker’s honoraria from Dainippon Sumitomo Eli Lilly, Janssen, Otsuka, Shionogi, Meiji, Pfizer, Novartis and Mochida.
References
- Smith DJ, Nicholl BI, Cullen B, et al. Prevalence and characteristics of probable major depression and bipolar disorder within UK biobank: cross-sectional study of 172,751 participants. PloS. one 8(11), e75362 (2013).
- Lima NN, do Nascimento VB, de Carvalho SM, et al. Childhood depression: a systematic review. Neuropsychiatr. Dis. Treat 9(1), 1417-1425 (2013).
- Judd LL, Schettler PJ, Coryell W, et al. Overt irritability/anger in unipolar major depressive episodes: past and current characteristics and implications for long-term course. JAMA. Psychiatry 70(11), 1171-1180 (2013).
- Katoh Y, Uchida S, Kawai M, et al. Onset of clinical effects and plasma concentration of fluvoxamine in Japanese patients. Biol. Pharm. Bull 33(12), 1999-2002 (2010).
- Irons J. Fluvoxamine in the treatment of anxiety disorders. Neuropsychiatr. Dis. Treat 1(4), 289-299 (2005).
- Omori IM, Watanabe N, Nakagawa A, et al. Efficacy, tolerability and side-effect profile of fluvoxamine for major depression: meta-analysis. J. Psychopharmacol 23(5), 539-550 (2009).
- Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr. Dis. Treat 5(1), 433-449 (2009).
- Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry. Clin. Neurosci 64(4), 341-357 (2010).
- Jiang C, Salton SR. The Role of Neurotrophins in Major Depressive Disorder. Trans. Neurosci 4(1), 46-58 (2013).
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol. Psychiatry 64(6), 527-532 (2008).
- Green, MJ, Matheson SL, Shepherd A, et al. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol. Psychiatry 16(9), 960-972 (2011).
- Pan W, Banks WA, Fasold MB, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37(12), 1553-1561 (1998).
- Licata SC, Shinday NM, Huizenga MN, et al. Alterations in brain-derived neurotrophic factor in the mouse hippocampus following acute but not repeated benzodiazepine treatment. PloS. one 8(12), e84806 (2013).
- Niles LP, Smith LJ. Tenn CC. Modulation of c-fos expression in the rat striatum by diazepam. Neurosci. Lett 236(1), 5-8 (1997).
- Salminen O, Lahtinen S, Ahtee L. Expression of Fos protein in various rat brain areas following acute nicotine and diazepam. Pharmacol. Biochem. Behav 54(1), 241-248 (1996).
- Morgan JI, Cohen DR, Hempstead JL, et al. Mapping patterns of c-fos expression in the central nervous system after seizure. Science 237(4811), 192-197 (1987).
- Hori H, Yoshimura R, Ueda N, et al. A case with occurring adverse effects when cross-over titration from fluvoxamine to paroxetine associated with increasing the plasma fluvoxamine level in major depressive disorder. World. J. Biol. Psychiatry 10(4 Pt 2), 620-622 (2009).
- Bocchio-Chiavetto L, Bagnardi V, Zanardini R, et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World. J. Biol. Psychiatry 11(6), 763-773(2010).
- Fernandes BS, Berk M, Turck CW, et al. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Mol. Psychiatry 19(7), 750-751 (2014).
- Yoshimura R, Kishi T, Suzuki A, et al. The brain-derived neurotrophic factor (BDNF) polymorphism Val66Met is associated with neither serum BDNF level nor response to selective serotonin reuptake inhibitors in depressed Japanese patients. Prog. Neuropsychopharmacol. Biol. Psych 35(4), 1022-1025 (2011).
- Fukuda K, Shoda T, Mima H, et al. Midazolam induces expression of c-Fos and EGR-1 by a non-GABAergic mechanism. Anesth. Analg 95(2), 373-378 (2002).
- Panford-Walsh R, Singer W, Ruttiger L, et al. Midazolam reverses salicylate-induced changes in brain-derived neurotrophic factor and arg3.1 expression: implications for tinnitus perception and auditory plasticity. Mol. Pharmacol 74(3), 595-604 (2008).
- Sarabi M, Perraud A, Mazouffre C, et al. Psychoactive drugs influence brain-derived neurotrophic factor and neurotrophin 4/5 levels in the serum of colorectal cancer patients. Biomed. Rep 6(1), 89-94 (2017).
- Zafra F, Castrén E, Thoenen H, et al. Lindholm DInterplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc. Natl. Acad. Sci 88(22), 10037-10041 (1991)
- Guilloux JP, Douillard-Guilloux G, Kota R, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 17(11), 1130-1142 (2012)
- Balu DT, Hoshaw BA, Malberg JE, et al. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain. Res 1211(1), 37-43 (2008)
- Geisel O, Hellweg R, Müller CA. Serum levels of brain-derived neurotrophic factor in alcohol-dependent patients receiving high-dose baclofen. Psychiatry. Res 240(1), 177-180 (2016)
- Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 62(1), 42-53 (2012)