Research Article - (2018) Volume 8, Issue 3
Differential Changes in Autonomic Functioning, HypothalamicÃÆÃÆÃââÃÆÃâÃâÃâ¬ÃÆÃâÃâÃâPituitaryÃÆÃÆÃââÃÆÃâÃâÃâ¬ÃÆÃâÃâÃâAdrenal Axis Activity and Inflammation during Melatonergic Antidepressant Treatment between Depressed Patients with and without Suicidal Ideation
- Corresponding Author:
- Hsin-An Chang
Department of Psychiatry, Tri-Service General Hospital, No. 325, Cheng-Kung Road, Sec. 2, Nei-Hu District, Taipei, 114, Taiwan, ROC
Tel: 011-886-2-8792-7220
Fax: 011-886-2-8792-7221
Abstract
Abstract
Objective:
The existing literature has not yet simultaneously examined the modification of autonomic functioning, hypothalamic–pituitary–adrenal (HPA) axis activity and inflammation following agomelatine treatment of depression. We investigated the changes in the three biological systems that are closely linked to cardiovascular risks among depressed patients with/without suicidal ideation (SI) following agomelatine treatment.
Methods
Thirty-two unmedicated depressed patients with current SI (SI+ group) and 28 without current/lifetime SI (SI− group) completed 6-week agomelatine treatment. None of them had any current or lifetime suicidal behavior before entering the study. The peripheral levels of cortisol, erythrocyte sedimentation rate (ESR) and high sensitivity C-reactive protein (hs- CRP), and heart rate variability (HRV) were measured at baseline and week 6. The Hamilton Depression Rating Scale (HAM-D) and the Columbia Suicide Severity Rating Scale (C-SSRS) were assessed at baseline, week 2, 4 and 6.
Results
Between-group analyses showed a significant treatment-by-group interaction for cortisol, ESR, hs-CRP, the ratio of cortisol to hs-CRP (COR/CRP ratio), variance (total HRV), low frequency (LF)-HRV, and high frequency (HF)-HRV. The results were driven by decreases in cortisol, hs- CRP and ESR levels and increases in total HRV, LF-HRV and HF-HRV in the SI+ group, coupled with an increase in hs-CRP levels and a decrease in COR/CRP ratio in the SI− group. Moreover, in the SI+ group the lessening of the intensity of SI independently predicted the reduction in hs-CRP levels.
Conclusions
Agomelatine treatment differentially modified biomarkers of cardiac risk between depressed patients with current SI and those without SI. The specificity and underlying mechanism of melatonergic antidepressant beneficially affecting markers of cardiac risk in only depressed patients with current SI merits further investigation. Peripheral hs-CRP levels may serve as a potential tool to monitor the fluctuation of SI in depressed patients under agomelatine treatment.
Keywords
Major depressive disorder, Agomelatine, Autonomic functioning, HPA axis, Inflammation, Suicide ideation
Introduction
Major depressive disorder (MDD) is associated with increased risk for cardiovascular diseases (CVDs) [1,2]. Pathophysiological changes in autonomic functioning, hypothalamic– pituitary–adrenal (HPA) axis activity and inflammation may partly explain the negative influence of MDD on cardiovascular health [3]. Specially, MDD is associated with reduced autonomic functioning, dysregulated HPA axis and elevated inflammation. All these MDDrelated biological dysregulations also constitute risk factors for CVDs. For example, reduced autonomic functioning, as index by low heart rate variability (HRV), is implicated with increased risks for cardiovascular morbidity [4] and mortality [5]. HPA axis dysregulation is more prevalent in patients with CVDs [6] and is associated with heightened risks for cardiovascular mortality [7]. Inflammation represents one mechanism that can directly accelerate atherosclerosis [8] while chronic, lowgrade systemic inflammatory response has been found to increase the risks for cardiovascular morbidity and mortality [9].
Whether pharmacological treatment of depression can mitigate the elevated CVD risks conveyed by depression is not well established so far although this contention has been supported by suggestions from studies in highrisk populations, i.e., cardiac patients [10,11]. For depressed patients without CVDs, it is very difficult to adequately power a study a priori given the great challenge in detecting infrequent cardiovascular outcomes and estimating response rates and predicted event rates. In this context, assessing the changes of biomarkers of cardiac risk following antidepressant treatment may be used as a proxy measure of whether antidepressants reduced CVD risks in depressed patients.
Previous literature has indicated that newer classes of antidepressants are capable of modulating HRV, HPA axis activity and inflammation in depressed patients. For example, treatment with both selective serotonin reuptake inhibitors (SSRIs) and serotonin-noradrenaline reuptake inhibitors (SNRIs) are associated with decreased HRV [12] and an elevated inflammatory marker [13]. Antidepressant treatment with SSRIs, but not SNRIs, reduces cortisol concentrations in depressed patients [14,15]. Agomelatine, a more recently introduced antidepressant, acts as a melatonergic (MT1/MT2) agonist as well as a 5-hydroxytryptamine (5-HT2C) antagonist [16]. Research information regarding the effects of agomelatine on the three biological systems is quite limited. Recently published studies indicated that depressed patients following agomelatine treatment showed increased HRV [17,18] and decreased inflammation [19,20]; however, little is known about the effect agomelatine on HPA-axis activity. Most importantly, the three biological systems are closely and reciprocally intertwined [21-23] whereas previous studies generally aimed at evaluating the effect of antidepressants on each system independently. To our knowledge, no study has simultaneously investigated the effects of agomelatine on the three biological systems in depressed patients.
Patients with MDD are hetereogenous groups and pathophysiological changes in autonomic functioning, HPA-axis activity and inflammation seem to differ between specific subgroups of depressed patients [3]. Our research team has recently verified that that depressed patients with current suicidal ideation (SI+ group) showed reduced HRV and elevated inflammation as compared to those without lifetime suicidal ideation (SI− group) and that in the SI+ group, the intensity of current suicidal ideation independently and positively correlated with the level of inflammation [24]. In the present study, we predict that agomelatine treatment exerts differential modifying effects on these markers of cardiac risk between the two subgroups of patients. Furthermore, we anticipate agomelatine treatment reduces these cardiac risk factors in the SI+ group only. Finally, we predict that following agomelatine treatment the changes in the intensity of current suicidal ideation will meaningfully correlate with the changes in the level of inflammation.
Methods
▪ Study design and participants
The Institutional Review Board of Tri-Service General Hospital approved the present study (No. of IRB approval: TSGHIRB-2-101-05-138) and all participants provided written informed consent. Unmedicated depressed patients between the ages of 20 and 65 were screened at the psychiatric outpatient clinic at the Tri-Service General Hospital in Taipei, Taiwan. Study inclusion criteria were (1) the diagnosis of nonpsychotic major depressive disorder (MDD); (2) having a score greater than 16 on the 17-item version of the Hamilton Depression Rating Scale (HAM-D) [25] rated by the treating psychiatrists (CCC and HAC); (3) either with current suicidal ideation (SI) or without any current/lifetime SI. All participants were interviewed by the treating psychiatrists using the Chinese version of the Modified Schedule of Affective Disorders and Schizophrenia-Lifetime [26] and fulfilled the DSM-IV criteria for MDD (Cohen’s Kappa value = 0.79) [27]. They underwent self-reported questionnaire screening, chart review, routine physical exam, electrocardiography, and relevant laboratory investigations. Each patient’s suicide risk was assessed by HAC using the Columbia Suicide Severity Rating Scale (C-SSRS), a semi-structured interview-based measure of current and past suicidal ideation and behavior [28]. Exclusion criteria were: (1) pregnancy or breastfeeding; (2) cancer, cardiovascular, respiratory, neurological, or endocrinological disorders that affect inflammatory status or autonomic functioning; (3) evidence of ongoing infection; (4) any current psychiatric comorbidity or history of substance dependence; (5) any current or lifetime suicidal behavior; and (6) treatment with antibiotics, antiinflammatory medications or any medications that affect autonomic functioning within 1 month prior to entering the study. Out of the 1102 consecutive depressed patients who were screened between September 2013 and July 2017, 60 were enrolled, and 1042 were excluded. The enrolled participants comprised 32 MDD patients with current SI (SI+ group) and 28 MDD patients without any current or lifetime SI (SI− group).
Both the SI+ and SI− groups received fixed daily dose of 25 mg agomelatine at 9:00 pm for a period of 6 weeks in an outpatient setting. With the exception of sedative-hypnotics on an as needed (prn) basis, no additional psychotropic medications or any medications that affect autonomic functioning were allowed. Patients in the SI+ and SI− groups were followed for 6 weeks and their cortisol levels and inflammatory markers in the blood, physiological markers were measured at baseline and week 6. For each patient, the same rater (who was blind to the biomarker results) administered the HAM-D and C-SSRS at study entry, week 2, 4 and 6. Treatment response was defined as the percentage reduction in HAM-D score. Responders were defined as patients showing at least a 50% decrease after treatment. All patients were reimbursed for transportation costs and time spent at each appointment attended.
▪ Clinical measures of suicide risk and chronic psychological stress
The Columbia Suicide Severity Rating Scale (C-SSRS) is a clinician-rated instrument frequently used in clinical trials, with acceptable validity and reliability [28]. The subscale of suicidal ideation (SI) contains items including a wish to be dead and the specificity of these thoughts, including whether they devise a specific plan and the intention for carrying out such a plan. The subscale of suicidal behavior (SB) categorizes actual, interrupted and aborted attempts to die, and preparatory behaviors, and for actual attempts, the potential or actual lethality or medical damage sustained in the attempt(s). The subscale of intensity of ideation (II) contains five items to measure the duration, frequency, controllability, deterrents, and reasons for suicide ideation. The items in SI and SB have yes/no responses. The items in II are rated on a 5-point Likert scale with the summed scores ranging from 5 to 25.
Chronic mental stress could have impact on vagal-immune pathway [29] and was evaluated using the Chinese version of the Perceived Stress Scale (PSS), a 14-item self-reported questionnaire with good internal consistency [30]. The participants assessed the degree to which individuals appraised their stressful events, and frequency and influence of everyday stressors during the past month [31]. Greater scores indicate more psychological stress (range 0–56).
▪ Measurements of peripheral cortisol, hs-CRP and ESR
Non-fasting blood was drawn between 8:30 and 12:00 AM. Blood samples collected in clot activator and sodium-heparin containing tubes were centrifuged to separate serum and plasma, respectively, and were stored at -80°C until assayed. The samples were analyzed at the biochemistry laboratory of Tri-Service General Hospital. The serum level of cortisol was measured using Advia Centaur automated immunoassays (Siemens AG, Erlangen, Germany) with interassay co-efficient of variations (CVs) of <10%. The serum concentration of high-sensitivity C-reactive protein (hs-CRP) was measured according to the manufacturer instructions for Beckman Coulter reagents on an IMMAGE 800 analyzer immunochemistry system. The minimum detectable concentration of hs-CRP was 0.01 mg/dL. Intra and inter-assay CVs were <10%. Erythrocyte sedimentation rate (ESR) was performed using an automatic analyzer determine the erythrocyte sedimentation rate (Sed Rat-Screener 20/II, Beckman, USA.). The ratio of cortisol to hs-CRP (COR/CRP) was calculated and was log-transformed. The COR/ CRP ratio can index the integrity of regulation between HPA axis and inflammatory response [32].
▪ Measurements of physiological markers
Our previous study has described the detailed procedures for measuring blood pressure, respiratory rate and HRV [24]. Briefly, tobacco smoking and caffeinated beverages were forbidden for 12 hours before measurements. A lead I electrocardiogram was recorded for 5 minutes while the subject lay quietly in a supine position in a warm and quiet room between 9 and 11 a.m. An HRV analyzer (SSIC, Enjoy Research Inc., Taipei, Taiwan) acquired, stored, and processed the ECG signals. The computer algorithm then identified each QRS complex and rejected each ventricular premature complex or noise according to its likelihood in a standard QRS template. Signals were recorded at a sampling rate of 512 Hz, using an 8-bit analogue-to-digital converter. Stationary R-R interval values were re-sampled and interpolated at a rate of 7.11 Hz to produce continuity in the time domain. The power spectral analysis was performed by using a non-parametric fast Fourier transformation. The direct current component was deleted, and a Hamming window was used to attenuate the leakage effect [33]. The power spectrum was then quantified into standard frequency-domain measurements including variance (variance of R-R-interval values), very low-frequency power (VLF, 0.003– 0.04 Hz), low-frequency power (LF, 0.04–0.15 Hz), high-frequency power (HF, 0.15–0.40 Hz), and the ratio of LF to HF power (LF/HF). Vagal and sympathetic control of HRV are jointly represented by LF, while vagal control of HRV is specifically represented by HF. The LF/HF ratio can mirror sympatho-vagal balance, with a larger LF/HF ratio indicating a greater predominance of sympathetic activity over cardiac vagal control. The definite physiological meaning of VLF component is still debated [34]. Pulse pressure is an important cardiovascular risk factor [35] that is independent of mean systolic blood pressure (Psystolic) and diastolic blood pressure (Pdiastolic) and can be calculated by the following formula: pulse pressure = Psystolic - Pdiastolic
▪ Statistical analysis
IBM SPSS Statistics 21.0 software (IBM SPSS Inc., Chicago, IL, USA) was used for analyses.
The Shapiro-Wilk test was used to check all data for deviations from a Gaussian distribution.
For the between-group comparisons of continuous variables, we used an independent-samples t-test for parametric variables, and the Mann-Whitney test for non-parametric variables. To evaluate between-group treatment effects on the serum biomarkers and physiological indices, repeated measures analyses of variance (RMANOVAs) were performed with the subgroup condition as the grouping factor (SI+ group versus SI− group), while pre- and post-treatment measures were used as repeated measures. Analysis of covariance adjusted for any significant differences in baseline characteristics known to affect the serum biomarkers and physiological indices. Significant effects were followed up to compare patients separately for each group condition. Spearman rank correlations were used to analyze the relationship between baseline levels of serum biomarkers and physiological indices, baseline HAM-D score and treatment response at week 2, 4 and 6 and the relationship between the changes in levels of serum biomarkers and physiological indices (from 0 to 6 weeks) and the concurrent changes in clinical rating scores over the same time period. Hierarchical regression analysis was used to examine the independent contribution of changes in the intensity of current suicidal ideation to the changes in hs-CRP levels, after adjusting for the control variables including age, gender, body mass index (BMI) and smoking [36,37]. The control variables relating to hs-CRP levels in univariate analysis (p<0.05) were entered into step 1 of the hierarchical regression analysis, when changes in the intensity of current suicidal ideation was the dependent variables. The α-level was set at 0.05 for each comparison unless otherwise stated. Bonferroni correction was applied to the problem of multiple comparisons.
Results
The SI+ and SI− groups showed no significant differences in demographic and clinical characteristics except the presence of current SI in the SI+ group (Table 1). The blood pressure, pulse pressure, respiratory rate and depressive symptoms before and after agomelatine treatment did not differ significantly between the patient groups (Table 1 and 2). None of the patients had any newly occurring suicidal ideation or suicidal behavior during the study period. In the SI+ group, the intensity of SI significantly dropped after 6-week agomelatine treatment. In both the SI+ and SI− groups, significant reductions in HAM-D scores were noted between the baseline and study endpoint. At week 6, there were more responders in the SI+ group (62.5%) than the SI− group (42.9%) , but the differences did not reach statistical significance (χ2=2.32, p= 0.13).
| All ( N=60 ) |
SI+ group ( N=32 ) |
SI-group ( N=28 ) |
p-valuesb | |
|---|---|---|---|---|
| Females (n, %) | 19 (31.70) | 8 (25.00) | 11 (39.30) | 0.24 |
| Age (years) | 30.03 ± 11.38 | 28.56 ± 10.65 | 31.71 ± 12.14 | 0.29 |
| Education level (years) | 14.52 ± 2.53 | 14.19 ± 2.61 | 14.89 ± 2.42 | 0.29 |
| Smokers (n, %) | 3 (5.00) | 1 (3.10) | 2 (7.10) | 0.59 |
| Alcohol use (drinks/day)a | 0.03 ± 1.18 | 0.03 ± 1.77 | 0.04 ± 0.19 | 0.93 |
| BMI (kg/m2) | 22.60 ± 3.56 | 22.37 ± 3.34 | 22.86 ± 3.84 | 0.60 |
| Weekly regular exercise (hours) | 0.10 ± 0.35 | 0.13 ± 0.42 | 0.07 ± 0.26 | 0.56 |
| Length of illness (months) | 15.40 ± 41.93 | 12.53 ± 28.74 | 18.68 ± 53.61 | 0.58 |
| Length of current episode (months) | 2.71 ± 4.3 | 1.67 ± 1.00 | 3.92 ± 6.09 | 0.06 |
| Lifetime number of MDEs | 1.17 ± 0.49 | 1.16 ± 0.37 | 1.18 ± 0.61 | 0.86 |
| Having used an sedative-hypnotic on an as-needed basis during the study period (n, %) | 22 (36.67) | 10 (31.30) | 12 (42.90) | 0.35 |
a Alcohol use is assessed with two items of the Alcohol Use Disorder Identification Test questionnaire (AUDIT) and is defined by the average frequency of drinking and the number of drinks consumed on a typical drinking day in the past year. From these items, we derived the average amount of alcoholic drinks per day, with one drink defined as a standard drink.
bComparison of SI+ and SI- subgroups.
Table 1: The demographic and clinical characteristics of the participants.
| All ( N=60 ) |
SI+ group ( N=32 ) |
SI-group ( N=28 ) |
p-valuesa | |
|---|---|---|---|---|
| Baseline SBP | 115.20 ± 13.85 | 114.25 ± 14.49 | 116.29 ± 16.28 | 0.58 |
| Baseline DBP | 76.10 ± 9.77 | 76.81 ± 10.13 | 75.29 ± 9.46 | 0.55 |
| Baseline pulse pressure | 39.10 ± 14.21 | 37.44 ± 12.26 | 41.00 ± 16.17 | 0.34 |
| Baseline RR | 13.26 ± 2.64 | 12.88 ± 2.59 | 13.69 ± 2.67 | 0.23 |
| PSS scores | 39.23 ± 7.33 | 38.19 ± 7.44 | 40.43 ± 7.16 | 0.24 |
| Initial HAM-D score | 38.55 ± 8.07b | 38.16 ± 7.90b | 39.00 ± 8.39 b | 0.69 |
| Initial intensity of SI | - | 9.78 ± 4.09c | - | - |
| After 6 weeks of agomelatine treatment | ||||
| SBP | 114.55 ± 14.00 | 113.69 ± 12.43 | 115.54 ± 15.78 | 0.61 |
| DBP | 75.40 ± 9.52 | 74.84 ± 9.11 | 76.03 ± 10.09 | 0.63 |
| Pulse pressure | 39.15 ± 12.45 | 38.84 ± 13.25 | 39.50 ± 11.69 | 0.84 |
| RR | 12.89 ± 2.63 | 13.15 ± 2.62 | 12.60 ± 2.67 | 0.43 |
| PSS scores | 24.20 ± 4.31 | 24.09 ± 4.70 | 24.32 ± 3.90 | 0.84 |
| HAM-D score | 19.03 ± 5.83b | 18.28 ± 5.78b | 19.89 ± 5.88 b | 0.29 |
| Change in HAM-D (%) | 50.73 ± 10.92 | 52.52 ± 8.72 | 48.66 ± 12.84 | 0.17 |
| Responders (%) | 53.3 | 62.5 | 42.9 | 0.13 |
| Intensity of SI | - | 6.34 ± 2.95c | - | - |
a Comparison of SI+ and SI- subgroups.
b p < 0.001, comparison of HAM-D score before and after treatment.
c p < 0.001, comparison of the score of intensity of SI before and after treatment
Table 2: Clinical characteristics of the patients before and after agomelatine treatment.
▪ Effects of agomelatine treatment on peripheral cortisol, hs-CRP, ESR and COR/ CRP ratio
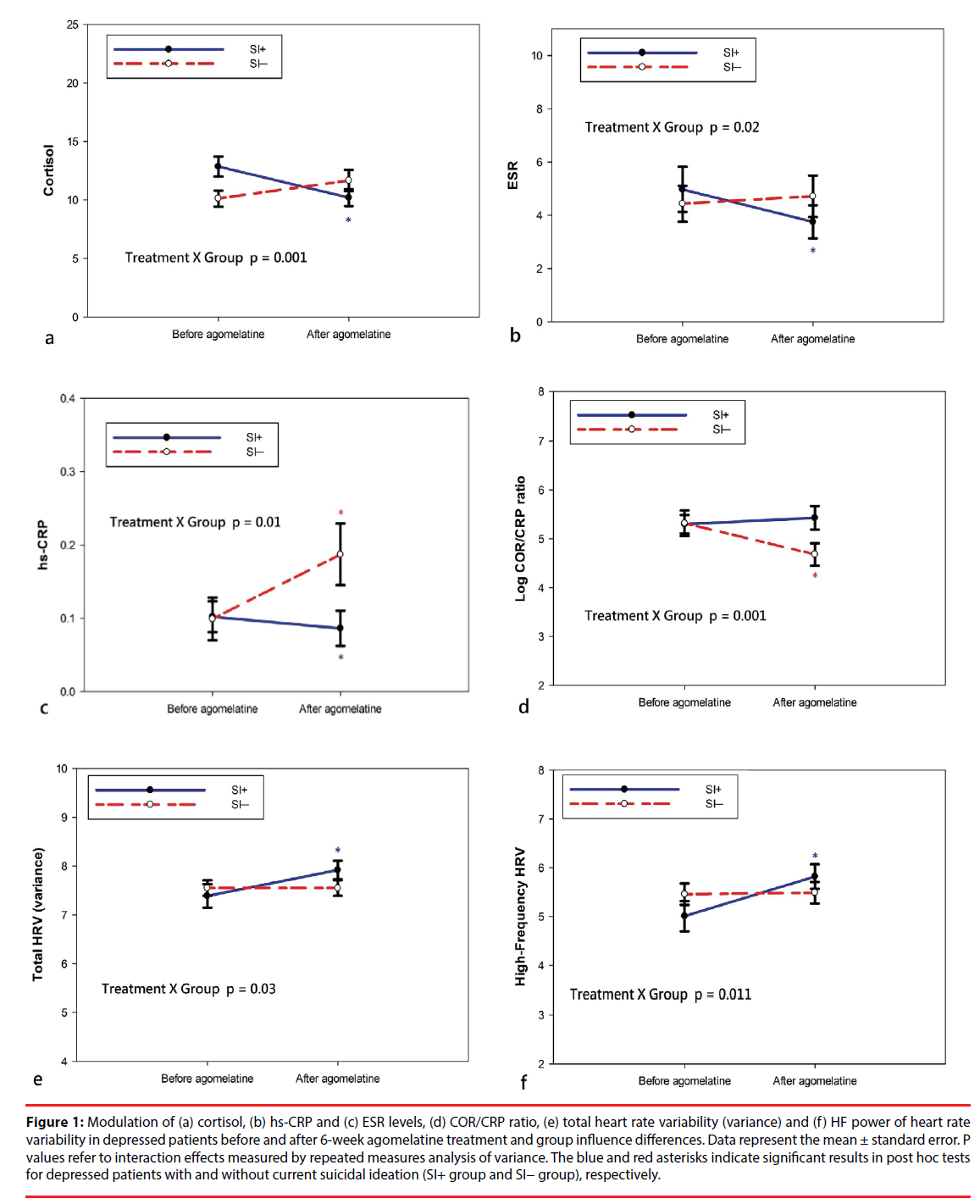
Table 3 describes the pre- and post-treatment changes in the blood levels of cortisol, hs-CRP and ESR and the COR/CRP ratio. Among all participants, the levels of cortisol, hs-CRP, ESR COR/CRP ratio did not significantly change following the 6-week treatment of agomelatine. For ANOVAs, treatment effect refers to preversus post-treatment conditions and group effect for the SI+ group versus the SI− group. There were significant treatment × group interaction effects for cortisol, hs-CRP, ESR and COR/CRP ratio (Figure 1). The results were due to a significant decrease in levels of cortisol (t = 3.08, p = 0.004), hs-CRP (W= 37.5, p = 0.002) and ESR (W= 49.0, p = 0.011) in the SI+ group and a significant increase in levels of hs-CRP (W= 27.0, p < 0.001) and a decrease in COR/CRP ratio (t = 4.71, p < 0.001) in the SI− group. Within the SI− group, there were no significant changes in levels of cortisol or ESR.
| Variables | SI+ group (N = 32) |
SI-group (N = 28) |
Statistics | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Treatment | Group | Treatment × Group | |
| Cortisol (µg/dL) | 12.82 ± 4.84 | 10.20 ± 4.09 | 10.12 ± 3.66 | 11.67 ± 4.80 | F1,58 = 0.78 p = 0.38 |
F1,58 = 0.41 p = 0.52 |
F1,58 = 11.80 p = 0.001 |
| hs-CRP, (mg/dl) | 0.06 (0.01−0.42) | 0.04 (0.01−0.72) | 0.04 (0.01−0.56) | 0.08 (0.02−0.74) | F1,58 = 3.14 p = 0.08 |
F1,58 = 1.81 p = 0.18 |
F1,58 = 6.83 p = 0.01 |
| ESR (mm/hour) | 3.0 (1.0−19.9) | 2.0 (1.0−17.0) | 2.5 (1.0−14.0) | 3.0 (1.0−16.0) | F1,58 = 2.28 p = 0.14 |
F1,58 = 0.05 p = 0.83 |
F1,58 = 5.92 p = 0.02 |
| Log COR/CRP ratio | 5.30 ± 1.10 | 5.43 ± 1.39 | 5.32 ± 1.39 | 4.68 ± 1.21 | F1,58 = 4.98 p = 0.03 |
F1,58 = 1.41 p = 0.24 |
F1,58 = 11.27 p = 0.001 |
| All (N = 60) | |||||||
| Pre | Post | Statistics | |||||
| Cortisol (µg/dL)a | 11.56 ± 4.51 | 10.89 ± 4.46 | t=1.02, p = 0.31 | ||||
| hs-CRP, (mg/dl)b | 0.04 (0.01−0.56) | 0.05 (0.01−0.74) | W=106.41, p = 0.24 | ||||
| ESR (mm/hour)b | 3.0 (1.0−19.0) | 2.5 (1.0−17.0) | W=349.50, p = 0.13 | ||||
| Log COR/CRP ratioa | 5.31 ± 1.23 | 5.08 ± 1.35 | t=1.86, p = 0.07 | ||||
aIndependent t test, mean and standard deviation.
bMann–Whitney test,median and interquartile range.
Table 3: Comparisons of cortisol, hs-CRP and ESR levels and COR/CRP ratio in depressed patients before and after agomelatine treatment and group influence differences.
Figure 1: Modulation of (a) cortisol, (b) hs-CRP and (c) ESR levels, (d) COR/CRP ratio, (e) total heart rate variability (variance) and (f) HF power of heart rate variability in depressed patients before and after 6-week agomelatine treatment and group influence differences. Data represent the mean ± standard error. P values refer to interaction effects measured by repeated measures analysis of variance. The blue and red asterisks indicate significant results in post hoc tests for depressed patients with and without current suicidal ideation (SI+ group and SI− group), respectively.
▪ Effects of agomelatine treatment on HRV
Table 4 presents the pre- and post-treatment changes in mean RR intervals and HRV indices. The ANOVAs showed significant treatment × group interaction effects for mean RR intervals and all HRV indices except for LF/HF ratio. The results were due to a significant increase in mean RR intervals (t = -2.99, p = 0.005), total HRV (t = -2.93, p = 0.006), VLF (t = -2.80, p = 0.001), LF (t = -3.56, p = 0.001) and HF (t = -4.19, p < 0.001) in the SI+ group. Within the SI− group, there were no significant changes in mean R-R intervals or any HRV indices. Among all participants, mean R-R intervals, total HRV, LF and HF significantly increased after 6-week agomelatine treatment.
| Variables | SI+ group (N = 32) |
SI-group (N = 28) |
Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Treatment | Group | Treatment × Group | |||||
| Mean RR intervals | 748.53 ± 159.86 | 843.53 ± 145.98 | 822.29 ± 136.51 | 818.64 ± 140.35 | F1,58 = 3.59 p = 0.06 |
F1,58 = 0.70 p = 0.41 |
F1,58 = 4.19 p = 0.045 |
||||
| Total HRV(variance) | 7.39 ± 1.35 | 7.92 ± 1.09 | 7.55 ± 0.84 | 7.55 ± 0.86 | F1,58 = 4.66 p = 0.04 |
F1,58 = 0.18 p = 0.68 |
F1,58 = 4.80 p = 0.03 |
||||
| VLF | 6.40 ± 1.41 | 6.99 ± 1.22 | 6.62 ± 0.95 | 6.57 ± 0.98 | F1,58 = 3.72 p = 0.06 |
F1,58 = 0.15 p = 0.70 |
F1,58 = 4.98 p = 0.03 |
||||
| LF | 5.87 ± 1.39 | 6.59 ± 1.23 | 6.17 ± 1.10 | 6.15 ± 1.19 | F1,58 = 5.64 p = 0.02 |
F1,58 = 0.06 p = 0.81 |
F1,58 = 6.24 p = 0.015 |
||||
| HF | 5.01 ± 1.74 | 5.82 ± 1.42 | 5.46 ± 1.18 | 5.49 ± 1.18 | F1,58 = 7.80 p = 0.007 |
F1,58 = 0.04 p = 0.85 |
F1,58 = 6.88 p = 0.011 |
||||
| LF/HF ratio | 0.97 ± 1.12 | 0.77 ± 0.74 | 0.71 ± 0.80 | 0.66 ± 0.91 | F1,58 = 0.88 p = 0.35 |
F1,58 = 0.95 p = 0.33 |
F1,58 = 0.35 p = 0.56 |
||||
| All (N = 60) | |||||||||||
| Pre | Post | Statistics | |||||||||
| Mean RR intervals | 777.95 ± 147.41 | 831.92 ± 142.72 | t = -2.24, p = 0.029, CI= −102.14 to −5.80 | ||||||||
| Total HRV(variance) | 7.46 ± 1.14 | 7.75 ± 1.00 | t = -2.24, p = 0.029, CI= −0.53 to −0.03 | ||||||||
| VLF | 6.50 ± 1.21 | 6.79 ± 1.13 | t = -2.01, p = 0.05, CI= −0.58 to −0.001 | ||||||||
| LF | 6.01 ± 1.26 | 6.38 ± 1.23 | t = -2.44, p = 0.018, −0.68 to −0.07 | ||||||||
| HF | 5.22 ± 1.51 | 5.67 ± 1.31 | t = -2.84, p = 0.006, −0.76 to −0.13 | ||||||||
| LF/HF ratio | 0.85 ± 0.99 | 0.72 ± 0.81 | t = 0.98, p = 0.33, −0.14 to 0.40 | ||||||||
Table 4: Comparisons of mean RR intervals and indices of heart rate variability during treatment and group influence differences.
▪ Correlation analysis
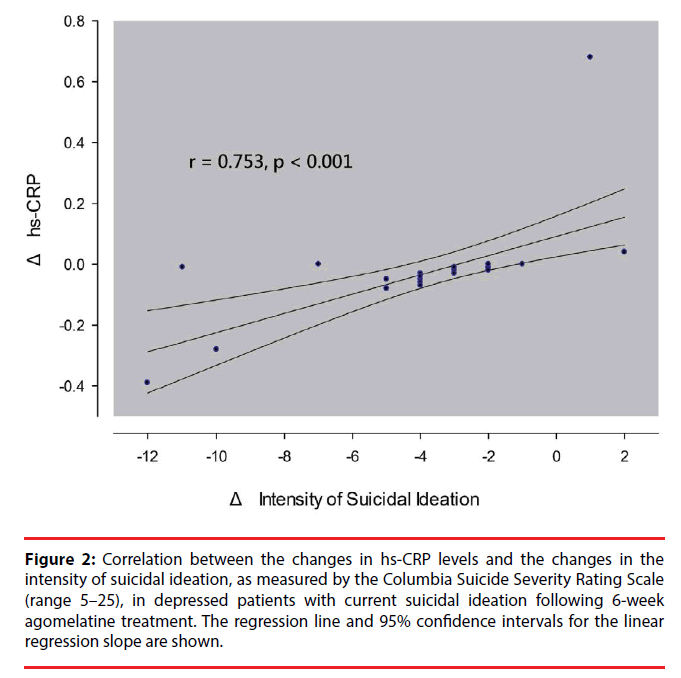
In all patients or in either group, baseline levels of cortisol, hs-CRP, ESR, COR/CRP ratio, mean RR intervals and HRV indices were not correlated with depressive symptom severity at baseline and treatment response at week 2, 4 and 6 (data not shown but available on request). Furthermore, the changes in all the biomarkers (from 0 to 6 weeks) were not correlated with the concurrent changes in depression severity over the same period of time (Supplementary information S1-3 Tables). In the SI+ group, the changes in hs-CRP levels were positively correlated with the changes in the intensity of SI (Figure 2 and supplementary information S1 Table). The correlation remained significant (r = 0.727, p < 0.001) when a subject with an extreme value was excluded from the analysis. The changes in the intensity of SI accounted for the additional variance (r2 = 31.6%, F = 14.74, p = 0.001) in the prediction of the changes in hs- CRP levels after adjusting for control variables of age, gender, smoking and BMI.
Figure 2: Correlation between the changes in hs-CRP levels and the changes in the intensity of suicidal ideation, as measured by the Columbia Suicide Severity Rating Scale (range 5–25), in depressed patients with current suicidal ideation following 6-week agomelatine treatment. The regression line and 95% confidence intervals for the linear regression slope are shown.
Discussion
A recent paper has indicated that agomelatine is safe for treating depression in patients with CVDs [38]. However, there is little evidence in the current literature regarding the cardiovascular risks of agomelatine in treating depressed patients without CVDs [39]. Several separate clinical studies have provided the evidence for the effects of agomelatine treatment on either autonomic functioning or inflammation in depressed patients. The data regarding the effect of agomelatine treatment on peripheral cortisol levels in depressed patients is lacking. What’s also insufficient is the data comparing the different changes in all these biomarkers after agomelatine treatment between specific subgroups of depressed patients. To our knowledge, this is the first study to investigate simultaneously the effects of agomelatine treatment on autonomic functioning, HPAaxis activity and inflammation in depressed patients with and without suicidal ideation. In the present study, agomelatine treatment differentially modified these markers of cardiac risk between the two subgroups of depressed patients. Agomelatine treatment reduced these cardiac risk factors only in the SI+ group but not in the SI− group. Furthermore, for the patients in the SI+ group following agomelatine treatment the greater decrease in the level of inflammation was associated with the greater decrease in the intensity of current SI. Overall, our study makes a practical advance in providing the information about the cardiovascular risk profiles of shortterm agomelatine treatment with respect to its effect on the three inter-correlated, cardiac risk- associated biological systems. In addition, through comparing suicidal and non-suicidal subgroups of depressed patients, our study explores a more comprehensive presentation of the phenomenology of depressive symptoms and the plausible links between cognitive, emotional and physiological dimensions of MDD, and brings a fresh perspective in choosing optimal pharmacological treatment for a subset of suicidal depressed patients that helps to reduce their potential cardiovascular risk factors.
There were differential changes in peripheral levels of cortisol, ESR and hs-CRP and COR/ CRP ratio and HRV indices during 6-week agomelatine treatment between the SI+ group and the SI− group. In the SI+ group, the parasympathetic tone and total HRV increased after agomelatine treatment. In line with this, two recent studies reported that 6-week treatment with agomelatine was associated with increased parasympathetic tone, and thus an overall reduction in total HRV in depressed patients [17,18]. The action of synergy between melatonergic receptors agonistic effect of agomelatine and its 5-HT2c receptor antagonistic effect was a proposed mechanism through which agomelatine increased vagal tone since both melatonergic and 5-HT2c receptors play a vital role in modulating vagal tone of the autonomic nervous system [40,41].
In the SI+ group, ESR and hs-CRP significantly dropped after 6-week agomelatine treatment. The effects of antidepressants on inflammation status in depressed patients are not yet conclusive [42-44] and may depend on the type of antidepressants or the subset of depressed population. Some researchers reported that SSRIs may decrease CRP in only males [45] or in both genders [46], while others reported that SSRIs may increase CRP [42]. There is only limited and inconsistent evidence for the effects of SNRIs on CRP levels, showing either no effect [47] or an increasing effect that is specific to male depressed patients [45]. Two recently published studies showed that 12-week treatment with agomelatine resulted in reduced levels of peripheral hs-CRP [19] or tumor necrosis factor-α [48] in depressed patients. The authors proposed that the ability of agomelatine to regulate circadian rhythms, which are known to have substantial effects on inflammatory responses [49], may be a potential mechanism through which agomelatine exerted anti-inflammatory effects.
After 6-week agomelatine treatment, the cortisol levels significantly dropped in the SI+ group. Studies have shown that other antidepressants can normalize overactivity of HPA axis in depressed patients [50-52] but our study is the first to report the effect of agomelatine on peripheral cortisol levels in depressed patients. It is known that HPA axis activity has crucial influence on inflammation status [53] and a dysregulated HPA axis may contribute to pro-inflammatory states [54]. Recent research indicated that the COR/CRP ratio helps to evaluate the integrity of the negative feedback loop between the HPA axis and inflammation system and a reduced COR/CRP ratio suggests an insufficient release of cortisol relative to increased inflammation and may be associated with depressogenic vulnerability [32]. Accordingly, agomelatine treatment did not change the COR/CRP ratio of patients in the SI+ group because there was a downward change in cortisol levels in response to a reduction in hs-CRP levels.
In the SI− group, agomelatine treatment did not produce any beneficial effects on markers of cardiac risk and what made things even worse, it increased the hs-CRP levels and decreased the COR/CRP ratio, i.e. elevated inflammatory response and an insufficient release of cortisol relative to increased inflammation. Moreover, the SI− group showed unchanged autonomic functioning following agomelatine treatment. As can be seen in Table 4, the increases in parasympathetic tone and total HRV for all depressed patients were driven by the vagotonic effect of agomelatine treatment in the SI+ group.
One possibility for these interesting findings is that agomelatine did have direct anti-inflammatory effect but there was a time-lag between the drug administration and its full-blown antiinflammatory effect. The significant antiinflammatory effect of agomelatine treatment might not show up until week 12 [19,48] and the inflammatory status of the depressed patients would continue upgrade before that time. Another possibility is that before the direct antiinflammatory effect of agomelatine showed up, the presence of agomelatine-associated increase in vagal tone might indirectly produce faster onset of anti-inflammatory effect. Previous literature indicated that vagus nerve directly controls the immune system via the cholinergic anti-inflammatory pathway, in which neurons in the motor division of the vagus nerve release acetylcholine and thereby dampen the inflammatory response [22]. Researchers have recently proved that vagus nerve stimulation can reduce inflammation in humans [55] by activating the cholinergic anti-inflammatorydependent mechanism [56]. Taken together, we therefore propose that failure of agomelatine to increase vagal activity in the SI− group, is a major determinant for the different changes in inflammatory response between the SI+ group and the SI− group. This conjecture has been supported by other researchers who noticed that CRP levels would increase in depressed patients whose vagal activity failed to increase following SSRIs treatment [57]. One might wonder why agomelatine treatment exerts vagotonic effect and thereby anti-inflammatory effect in the SI+ group but not the SI− group. It is possible that compared to the SI− group, the SI+ group tends to have a pathophysiological profile of dysregulation in both autonomic functioning and inflammation [24]. In fact, some researchers argued that MDD with SI is a specific subtype of MDD that is characterized by impulsive behavior [58,59] and patients of this subtype consistently exhibit recurrent SI across their depressive episodes [60,61]. Other researchers further suggested that reduced HRV [62,63] and low-grade inflammation [64] are trait markers for suicidal depression. In this context, patients of the SI+ depression subtype might especially benefit from treatment with antidepressants having potential vagotonic and/ or anti-inflammatory properties. In addition to the profits of reducing markers of cardiac risks, patients in the SI+ group tended to have higher response rate than the SI− group (Table 2). Future studies with larger sample sizes are necessary to verify this observation.
In the SI+ group following agomelatine treatment, the greater reduction in hs-CRP levels was associated with the greater reduction in the intensity of SI, independently from the control variables. This result was reinforced by our [24] and other research team [65] pointing out the positive correlation between the suicidal risk and serum hs-CRP levels among depressed patients. Similarly, O’Donovan et al. reported that depressed patients with greater SI had higher inflammation than those with lesser SI [66]. In addition, the positive correlation between the changes in suicidal risk and the changes in inflammation status is not simply an epiphenomenon, but also provides an alternative putative mechanism of action for electroconvulsive therapy in relieving the expression of suicidal ideation [67]: presumably via long-term down-regulation of immunoinflammatory activation [68,69]. Taken together, the finding reported herein may have clinical importance in suicide risk assessment. The application of measuring peripheral hs- CRP levels in monitoring the fluctuation of depressed patients’ suicide risk when they were under antidepressant treatment merits further investigation.
Depressed populations are heterogeneous. More homogenous subtypes help to identify the differences in pathophysiological profiles and pave the way for personal medicine. For example, the melancholic depression subtype showed more HPA axis hyperactivity while the atypical depression subtype showed heightened inflammation response [3]. The SI+ group is a potential clinical subtype of depression [58,70], showing the pathophysiological characteristics of decreased vagal tone and increased inflammation [24]. This subgroup of depressed patients may represent a new target for personalized medicine (i.e., treating them with an antidepressant with potential vagotonic and/or anti-inflammatory properties such as agomelatine) and should be confirmed in future large-scale studies.
Limitations
Our study has several limitations. First, the modest sample size limits the inferential power of the present study. Second, the “statistically significant” differences reported herein do not necessarily result in clinical significance. Specifically, elevated peripheral level of hs- CRP is a well-known biomarker that predicts future cardiovascular events in healthy subjects and the levels of <0.1, 0.1-0.3, and > 0.3 mg/ dl represents lower, moderate and higher risk of coronary artery heart disease, respectively [45,71]. Although hs-CRP levels significantly elevated following agomelatine treatment in the SI− group, there was a mild increase in patients whose hs-CRP concentrations fell into the range of moderate to high risk (from 6/28 to 10/28). Third, the present study has a relatively short follow-up period. Clinical improvement in MDD might also influence the changes in autonomic functioning, HPA-axis activity and inflammation. It should be interpreted with caution whether the changes in the three biological systems derived from agomelatine treatment. Finally, we did not establish a diurnal pattern of saliva cortisol levels by frequently sampling during the day and night but measured serum cortisol level once in the morning, which is not a reliable indicator of HPA function.
Conclusion
Our study points out the differential modifications of autonomic functioning, HPA-axis activity and inflammation following agomelatine treatment between depressed patients with and without SI. The lessening of the intensity of SI in depressed patients with SI is independently responsible for the reduction in inflammatory response. These findings have important clinical implications for cardiovascular risk in depressed patients under antidepressant treatment given the connections between the three biological systems and cardiovascular risk. Future research is necessary to shed light on the specificity and mechanism of agomelatine treatment that beneficially affects markers of cardiac risk in depressed patients with current SI. It also warrants further investigation into whether the use of peripheral hs-CRP levels aids in monitoring the suicide risk of depressed patients under antidepressant treatment.
Acknowledgments
This study was supported in part by grants from the Ministry of Science and Technology of the Taiwanese Government (MOST-103-2314-B-016-021), the Tri-Service General Hospital (TSGH-C106-110), and the National Defense Medical Research (MAB- 106-023).
Statement of Interest
The authors have no conflicting interests to declare.
References
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom. Med 66(6), 802-813 (2004).
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur. Heart. J 27(23), 2763-2774 (2006).
- Penninx BW. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci. Biobehav. Rev 74(Pt B), 277-286 (2017).
- Hillebrand S, Gast KB, de Mutsert R, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace 15(5), 742-749 (2013).
- Maheshwari A, Norby FL, Soliman EZ, et al. Low Heart Rate Variability in a 2-Minute Electrocardiogram Recording Is Associated with an Increased Risk of Sudden Cardiac Death in the General Population: The Atherosclerosis Risk in Communities Study. PLoS. One 11(8), e0161648 (2016).
- Bradley SM, Rumsfeld JS. Depression and cardiovascular disease. Trends. Cardiovasc. Med 25(7), 614-622 (2015)
- Kumari M, Shipley M, Stafford M, et al. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J. Clin. Endocrinol. Metab 96(5), 1478-1485 (2011).
- Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature 487(7407), 325-329 (2012).
- Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375(9709), 132-140 (2010).
- Glassman AH, O'Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 288(6), 701-709 (2002).
- Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch. Intern. Med 170(7), 600-608 (2010).
- Kemp AH, Brunoni AR, Santos IS, et al. Effects of depression, anxiety, comorbidity, and antidepressants on resting-state heart rate and its variability: an ELSA-Brasil cohort baseline study. Am. J. Psychiatry 171(12), 1328-1334 (2014).
- Chang HH, Lee IH, Gean PW, et al. Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain. Behav. Immun 26(1), 90-95 (2012).
- Piwowarska J, Chimiak A, Matsumoto H, et al. Serum cortisol concentration in patients with major depression after treatment with fluoxetine. Psychiatry. Res 198(3), 407-411 (2012).
- Scharnholz B, Weber-Hamann B, Lederbogen F, et al. Antidepressant treatment with mirtazapine, but not venlafaxine, lowers cortisol concentrations in saliva: a randomised open trial. Psychiatry. Res 177(1-2), 109-113 (2010).
- Demyttenaere K. Agomelatine: a narrative review. Eur. Neuropsychopharmacol 21 Suppl 4, S703-709 (2011).
- Yeh TC, Kao LC, Tzeng NS, et al. Heart rate variability in major depressive disorder and after antidepressant treatment with agomelatine and paroxetine: Findings from the Taiwan Study of Depression and Anxiety (TAISDA). Prog. Neuropsychopharmacol. Biol. Psychiatry 64(1), 60-67 (2016).
- Chang CC, Tzeng NS, Yeh CB, et al. Effects of depression and melatonergic antidepressant treatment alone and in combination with sedative-hypnotics on heart rate variability: Implications for cardiovascular risk. World. J. Biol. Psychiatry, 1-11 (2017).
- De Berardis D, Fornaro M, Orsolini L, et al. Effect of agomelatine treatment on C-reactive protein levels in patients with major depressive disorder: an exploratory study in "real-world," everyday clinical practice. CNS. Spectr, 1-6 (2016).
- Gupta K, Gupta R, Bhatia MS, et al. Effect of Agomelatine and Fluoxetine on HAM-D Score, Serum Brain-Derived Neurotrophic Factor, and Tumor Necrosis Factor-alpha Level in Patients With Major Depressive Disorder With Severe Depression. J. Clin. Pharmacol, (2017).
- Johnson EO, Kamilaris TC, Chrousos GP, et al. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci. Biobehav. Rev 16(2), 115-130 (1992).
- Tracey KJ. The inflammatory reflex. Nature 420(6917), 853-859 (2002).
- Bellavance MA, Rivest S. The HPA - Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front. Immunol 5, 136 (2014).
- Chang CC, Tzeng NS, Kao YC, et al. The relationships of current suicidal ideation with inflammatory markers and heart rate variability in unmedicated patients with major depressive disorder. Psychiatry Res (2017).
- Halaris A. Inflammation, heart disease, and depression. Curr. Psychiatry. Rep 15(10), 400 (2013).
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch. Gen. Psychiatry 35(7), 837-844 (1978).
- Chang CC, Lu RB, Chen CL, et al. Lack of association between the norepinephrine transporter gene and major depression in a Han Chinese population. J. Psychiatry. Neurosci 32(2), 121-128 (2007).
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 168(12), 1266-1277 (2011).
- Visnovcova Z, Mokra D, Mikolka P, et al. Alterations in vagal-immune pathway in long-lasting mental stress. Adv. Exp. Med. Biol 832, 45-50 (2015).
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal. of. Health. and Social. Behavior 24(4), 385-396 (1983).
- Chu LC, Kao HSR. The moderation and meditation experience and emotional intelligence on the relationship between perceived stress and negative mental health. Chinese. J. Psychol 47(1), 157-179 (2005).
- Suarez EC, Sundy JS, Erkanli A. Depressogenic vulnerability and gender-specific patterns of neuro-immune dysregulation: What the ratio of cortisol to C-reactive protein can tell us about loss of normal regulatory control. Brain. Behav. Immun 44(1), 137-147 (2015).
- Kuo TB, Lin T, Yang CC, et al. Effect of aging on gender differences in neural control of heart rate. Am. J. Physiol 277(6 Pt 2), H2233-2239 (1999).
- Lombardi F. Clinical implications of present physiological understanding of HRV components. Card .Electrophysiol. Rev 6(3), 245-249 (2002).
- Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation 100(4), 354-360 (1999).
- Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA 282(22), 2131-2135 (1999).
- Lakoski SG, Cushman M, Criqui M, et al. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am. Heart. J 152(3), 593-598 (2006).
- Medvedev VE. Agomelatine in the treatment of mild-to-moderate depression in patients with cardiovascular disease: results of the national multicenter observational study PULSE. Neuropsychiatr. Dis. Treat 13, 1141-1151 (2017).
- Carvalho AF, Sharma MS, Brunoni AR, et al. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom 85(5), 270-288 (2016).
- Nishiyama K, Yasue H, Moriyama Y, et al. Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. Am. Heart. J 141(5), E9 (2001).
- Jordan D. Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp. Physiol 90(2), 175-181 (2005).
- Eyre HA, Lavretsky H, Kartika J, et al. Modulatory Effects of Antidepressant Classes on the Innate and Adaptive Immune System in Depression. Pharmacopsychiatry 49(3), 85-96 (2016).
- Hiles SA, Baker AL, de Malmanche T, et al. Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis. Psychol. Med 42(10), 2015-2026 (2012).
- Hamer M, Batty GD, Marmot MG, et al. Anti-depressant medication use and C-reactive protein: results from two population-based studies. Brain. Behav. Immun 25(1), 168-173 (2011).
- Vogelzangs N, Duivis HE, Beekman AT, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl. Psychiatry 2, e79 (2012).
- Chavda N, Kantharia ND, Jaykaran. Effects of fluoxetine and escitalopram on C-reactive protein in patients of depression. J. Pharmacol. Pharmacother 2(1), 11-16 (2011).
- Piletz JE, Halaris A, Iqbal O, et al. Pro-inflammatory biomakers in depression: treatment with venlafaxine. World J Biol Psychiatry 10(4), 313-323 (2009).
- Gupta K, Gupta R, Bhatia MS, et al. Effect of Agomelatine and Fluoxetine on HAM-D Score, Serum Brain-Derived Neurotrophic Factor, and Tumor Necrosis Factor-alpha Level in Patients With Major Depressive Disorder With Severe Depression. J. Clin. Pharmacol 57(12), 1519-1526 (2017).
- Kizaki T, Sato S, Shirato K, et al. Effect of Circadian Rhythm on Clinical and Pathophysiological Conditions and Inflammation. Critical. Reviews. In. immunology 35(4), 261-275 (2015).
- Nikisch G, Mathe AA, Czernik A, et al. Long-term citalopram administration reduces responsiveness of HPA axis in patients with major depression: relationship with S-citalopram concentrations in plasma and cerebrospinal fluid (CSF) and clinical response. Psychopharmacology (Berl) 181(4), 751-760 (2005).
- Himmerich H, Binder EB, Kunzel HE, et al. Successful antidepressant therapy restores the disturbed interplay between TNF-alpha system and HPA axis. Biol. Psychiatry 60(8), 882-888 (2006).
- Schule C, Baghai TC, Eser D, et al. Effects of mirtazapine on dehydroepiandrosterone-sulfate and cortisol plasma concentrations in depressed patients. J. Psychiatr. Res 43(5), 538-545 (2009).
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332(20), 1351-1362 (1995).
- Straub RH, Buttgereit F, Cutolo M. Alterations of the hypothalamic-pituitary-adrenal axis in systemic immune diseases - a role for misguided energy regulation. Clinical. And. Experimental. Rheumatology 29(5 Suppl 68), S23-31 (2011).
- Koopman FA, Chavan SS, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci .U S A 113(29), 8284-8289 (2016).
- Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J. Physiol 594(20), 5781-5790 (2016).
- Dawood T, Lambert EA, Barton DA, et al. Specific serotonin reuptake inhibition in major depressive disorder adversely affects novel markers of cardiac risk. Hypertension research : official journal of the Japanese Society of Hypertension 30(4), 285-293 (2007).
- Oquendo MA, Baca-Garcia E, Mann JJ, et al. Issues for DSM-V: suicidal behavior as a separate diagnosis on a separate axis. Am. J. Psychiatry 165(11), 1383-1384 (2008).
- Booij L, Swenne CA, Brosschot JF, et al. Tryptophan depletion affects heart rate variability and impulsivity in remitted depressed patients with a history of suicidal ideation. Biol. Psychiatry 60(5), 507-514 (2006).
- Williams JM, Crane C, Barnhofer T, et al. Recurrence of suicidal ideation across depressive episodes. J. Affect. Disord 91(2-3), 189-194 (2006)
- Antypa N, Van der Does AJ, Penninx BW. Cognitive reactivity: investigation of a potentially treatable marker of suicide risk in depression. J. Affect. Disord 122(1-2), 46-52 (2010).
- Chang HA, Chang CC, Chen CL, et al. Major depression is associated with cardiac autonomic dysregulation. Acta. Neuropsychiatr 24(6), 318-327 (2012).
- Chang HA, Chang CC, Chen CL, et al. Heart rate variability in patients with fully remitted major depressive disorder. Acta. Neuropsychiatr 25(1), 33-42 (2013).
- Ekinci O, Ekinci A. The connections among suicidal behavior, lipid profile and low-grade inflammation in patients with major depressive disorder: a specific relationship with the neutrophil-to-lymphocyte ratio. Nord. J. Psychiatry 71(8), 574-580 (2017).
- Priya PK, Rajappa M, Kattimani S, et al. Association of neurotrophins, inflammation and stress with suicide risk in young adults. Clin .Chim. Acta 457, 41-45 (2016).
- O'Donovan A, Rush G, Hoatam G, et al. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress. Anxiety 30(4), 307-314 (2013).
- Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am. J. Psychiatry 162(5), 977-982 (2005).
- Guloksuz S, Rutten BP, Arts B, et al. The immune system and electroconvulsive therapy for depression. J. ECT 30(2), 132-137 (2014).
- Yrondi A, Sporer M, Peran P, et al. Electroconvulsive therapy, depression, the immune system and inflammation: A systematic review. Brain. Stimul (2017).
- Joiner TE. Why people die by suicide: Cambridge, MA Harvard University, (2005).
- Molteni R, Macchi F, Zecchillo C, et al. Modulation of the inflammatory response in rats chronically treated with the antidepressant agomelatine. Eur. Neuropsychopharmacol 23(11), 1645-1655 (2013).