Research Paper - (2018) Volume 8, Issue 5
Determinants of Progression and Mortality in Alzheimers disease: A Systematic Review
- Corresponding Author:
- Dr. Pedro J Modrego
Department of Neurology, Hospital Universitario Miguel Servet, Zaragoza-50009, Spain
Abstract
Purpose
Neuropsychiatric symptoms (NPS) have been regarded as predictors of poor outcomes, and the use of neuroleptics as well, but with contradictory results. The objective of this article is to perform a systematic review of published works dealing with progression and survival in AD.
Methods
We searched articles aimed at determining the factors predicting faster cognitive decline and mortality in AD, published from January 1987 to April, 2018. We gave priority to prospective and retrospective cohorts analysing the influence of gender, NPS, neuropsychological predictors, co morbidities, and the use of neuroleptics/ antidepressants. The data-sources were 42 prospective cohorts including 24,711 patients; Hazard ratios/relative risks of mortality and faster cognitive decline were obtained for NPS and the use of psychotropic drugs. The risk of mortality for neuroleptics and ChEI was also obtained from 7 retrospective cohorts totalling 242,013 patients followed up.
Results
High levels of NPS were predictors of both mortality and faster cognitive decline Considered in isolation, agitation/aggression, hallucinations, and depression predicted mortality. However the results for apathy and psychosis/delusions varied across the studies. According to prospective cohort studies there is no clear evidence that neuroleptics increase mortality. The use of neuroleptics is associated with faster cognitive deterioration. Conventional neuroleptics for NPS did not result in lower mortality rates than the atypical ones.
Conclusions
The presence of NPS is associated with poor outcomes in AD such as mortality and greater cognitive decline. Atypical neuroleptics do not shorten survival but are associated with faster cognitive decline
Keywords
Alzheimer disease, Neuropsychiatric symptoms, Mortality, Neuroleptic use
Introduction
Alzheimer’s disease (AD) is a functionally and mentally devastating disease but the clinical course varies among patients, with long periods of stabilisation in many patients and rapid progression and shorter survival in others. Life expectancy varies from 3 to 10 years ant it depends on the age at onset and many other variables [1]. Little is known about the neurobiological basis of these differences and about predictors of rapid progression and mortality. Co-morbidities are frequent in AD patients and tend to increase in frequency and severity over time, especially depression, fluid/electrolyte disorders, weight loss, and psychosis, which may predict worse outcomes in a nationwide inpatient sample [2].
Neuropsychiatric symptoms such as depression, apathy, aggression, and psychosis, have emerged as predictors of faster cognitive decline, disability, and shorter survival [3,4]. The biological basis of these symptoms is poorly understood, but psychological, social, functional, and environmental factors play a role in its development [5]. Depression is one of the most studied neuropsychiatric co-morbid condition in AD, and that appears in around 50% of patients [6], it does not seem to arise from dysfunction of serotoninergic or noradrenergic systems, but from dysfunction in the glutamatergic system [7], or as a consequence of oxidative stress [8]. Anxiety is also very common in AD patients, up to 70% of them may have anxiety, and 54% had anxiety plus depression [9]. Delusions, agitation, aggression, hallucinations, sleep disturbances are stressful symptoms for patients and caregiver with increasing prevalence overtime. In Table 1 are reported the frequencies of the different neuropsychiatric symptoms according to a systematic review of 48 eligible works [10].
| NEUROPSYCHIATRIC SYMPTOM | POOLED PREVALENCE |
|---|---|
| Apathy | 49%; 95% CI: 41-57% |
| Depression | 42%; 95% CI: 37-46 |
| Agitation/Aggression | 40%; 95% CI: 33-46 |
| Anxiety | 39%; 95% CI: 32-46 |
| Sleep Disorders | 39%; 95% CI: 30-47 |
| Irritability | 36%; 95% CI: 31-41 |
| Apetite disorder | 34%; 95% CI: 27-41 |
| Aberrant motor behavior | 32%; 95% CI: 25-38 |
| Delusions | 31%; 95% CI: 27-35 |
| Disinhibition | 17%; 95% CI: 17-21 |
| Hallucinations | 16%; 95% CI: 13-18 |
| Euphoria | 7%; 95% CI: 5-9 |
Table 1: Prevalence of behavioural symptoms in AD [10].
Neuropsychiatric/behavioural symptoms have been associated with worse outcome in AD and other dementias [3]. They led to institutionalization in many cases as shown by a large cohort of 1145 patients [11]. Not all symptoms are equally distressing for patients and caregivers. Agitation/aggression is the most distressful behavioural symptoms with possible harm for patients and caregivers, and leading to the use of neuroleptics [12]. Although not the most frequent, delusions and hallucinations are very disturbing symptoms for which age, gender and education level are not predictive; they that may appear in up to 51% of patients at 4 years [13] Until recently, there was no clear consensus on the definition of agitation; but, according to the International Psychogeriatric Association, agitation is defined as emotional distress associated with at least one of the following symptoms: excessive verbal activity, verbal aggression, or physical aggression [14].
Given the high prevalence of AD and related behavioural symptoms, as well as the big impact on the healthcare system costs and caregiver burden, the objective of this review is to analyse in a comprehensive manner the determinant factors of survival and faster cognitive decline in AD, with especial focus on neuropsychiatric symptoms, and the influence of antipsychotics and antidepressants on these outcomes.
Methods/Literature Search
The main questions to be answered were: a) Does the presence of neuropsychiatric symptoms predict higher mortality and/or faster cognitive decline in AD?; b) Is the use of neuroleptics associate with shorter survival/faster cognitive decline in AD patients? c) Are there other predictors of mortality and poorer outcomes in AD?
For analysing data sources, we have followed the PRISMA guidelines. We have searched in PubMed and Cochrane Library articles focused in predictors of mortality/survival, faster cognitive decline in AD: co-morbidities, vascular risk factors, gender, extrapyramidal symptoms, neuropsychiatric symptoms, use of psychotropic drugs, and genetic factors, published from January 1985 to April 2018. We gave preference to the studies with the highest level of evidence for prognosis in the following order: prospective cohorts, retrospective cohorts, meta-analysis from clinical trials using neuroleptics or antidepressants, case-control studies, and previous systematic reviews. We used the levels of clinical evidence for prognosis studies of the North America Spine Society, 2005.
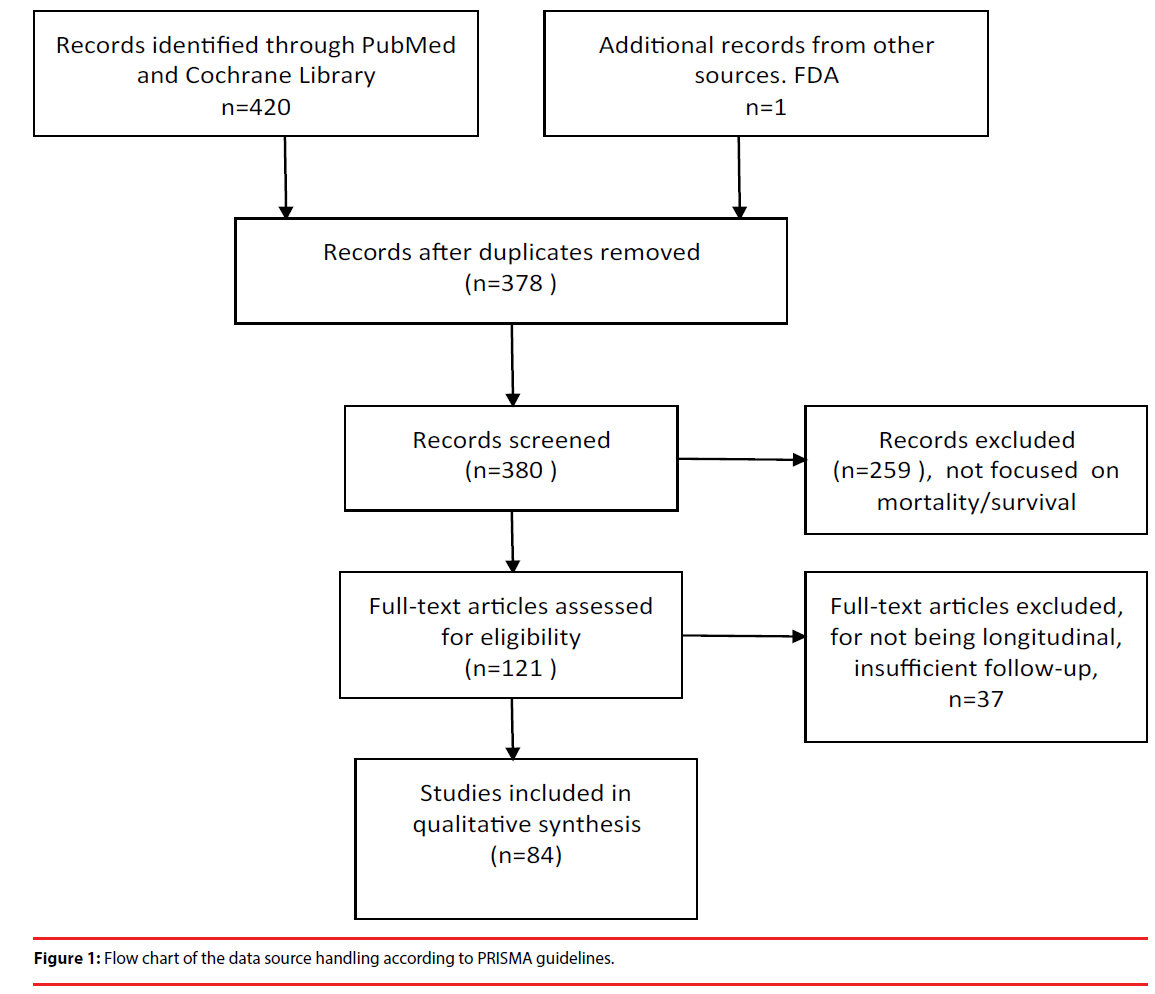
Initially we identified 426 articles dealing with mortality/survival, faster cognitive decline, and neuroleptics related mortality. After reading the abstracts we selected 121 articles pertinent to this review, and finally 84 of them offered relevant information, and were used for the systematic review (Figure 1).
The quality of the studies was based on the clarity of the selection criteria of the patients, the estimates of the risk for each variable studied: adjusted Hazard Ratios or Relative Risks, and the one-year minimum follow-up period. Among these studies, 42 were prospective cohorts comprising a total of 24,711 patients followedup (level I of evidence); 3 cohorts with 1177 patients focused on the use of ChEI drugs and outcomes; 7 cohorts with 2846 patients focused on NPS and the use of drugs as predictors of unfavourable outcomes, and 31 cohorts with 20,688 patients dealing with NPS and other predictors of death or faster cognitive decline in AD. Then, we revised 10 retrospective cohorts totalling 244,477 patients level II of evidence); 2 dealt with non-pharmacological predictors of cognitive decline and included 23,945 AD patients; 1 with ChEI use and mortality in 2464 patients, and 7 dealt with mortality and other side-effects in 218,068 patients. The articles selected have been published in peer reviewed journals, and preferred in English, but without discarding valid papers in other languages. We did not include unpublished cohort studies.
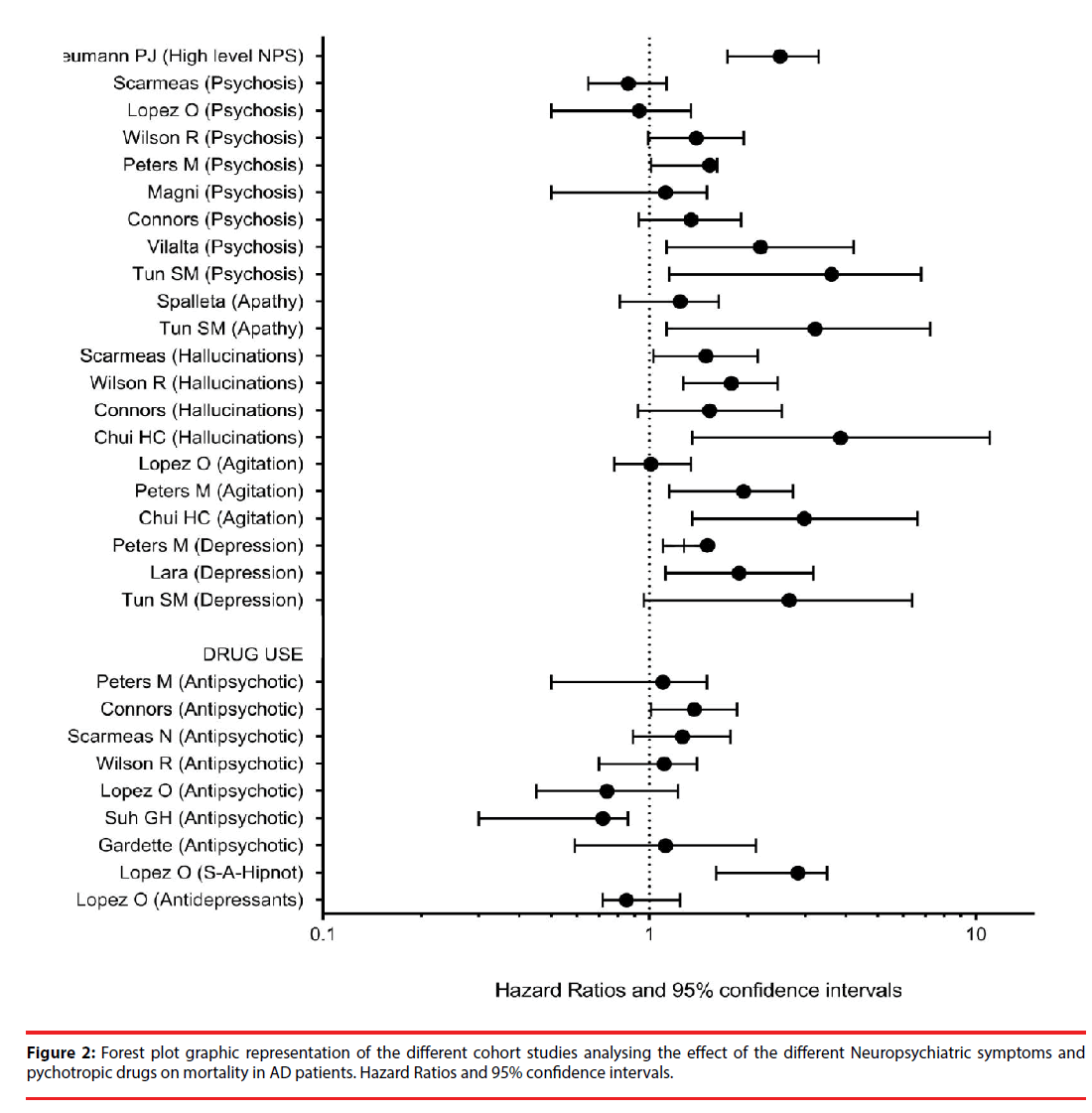
We analysed the predictive power of a series of factors on survival/mortality, and the progression of cognitive decline; these factors were: gender, vascular risk factors, extrapyramidal signs, neuropsychiatric symptoms, the use of psychotropic drugs, genetic factors, and other predictors. Especial emphasis was put on neuropsychiatric symptoms and the use of neuroleptics. The main statistical parameter estimated was the adjusted hazard ratio or relative risk for each predictor. Meta-analysis was not possible because of the lack of complete datasets. Despite this caveat, we have displayed the HR and 95% CI of the different prospective cohorts in a forest plot graphic for better distinguishing the estimations and differences among the cohorts analysed.
Results
▪ Demographic factors
In Table 2 are given the predictive factors of poorer outcomes in AD found in the different papers revised. Among demographic variables, there are four predictors that appeared consistently across many cohort studies: male gender [15-23] and extrapyramidal signs [24-29], which are associated with both shorter survival and faster cognitive decline. Only in one study the female gender was associated with poorer outcome [30]. In the large retrospective cohort study comprising 5831 men and 17918 women, the shorter survival in men was explained by the increased number of co-morbidities in this group (cancer, arrhythmia, obstructive pulmonary disease, and Parkinson’s disease [21]. High educational level appeared also as an indicator of worse outcomes [22,31-33]. Vascular risk factors were associated with poorer outcomes in some studies [33-36] but not in another one [32].
| 1. psychiatric symptoms (Tables 3 and 4). 2. Language and executive function impairment in early phases [16,32] 3. High CSF t-tau and p-tau levels [69] 4. Low plasma levels of Abeta40 and Abeta42, and C-reactive protein levels [70] 5. Sociodemographic factors: - Higher educational level [22,31-33]. - Male gender [11,15-23,58]. - Female gender [30]. 6. Comorbidities, vascular risk factors (AHT, dyslipemia, diabetes) [33-36]. 7. Impairment of daily living activities [71]. 8. Caregivers attitude [72] 9. Genetic factors (ApoE4 genotype with contradictory results) [38-40]. 10. Severity of cognitive impairment at baseline [23,61,63,73] 11. Cortical atrophy on MR-based volumetry [75]. 12. Presence/absenc of treatment with ChEI [76-79]. 13. Signs of parkinsonism [26-29,43,61] 14. Nutritional status [30,74] |
Table 2: Predictors of poor outcomes in AD (mortality, faster cognitive decline, or worse functional status.
▪ Genetic factors
The APOE genotype has been used as marker of risk for AD, but it is also highly prevalent in MCI patients, and normal subjects. The APOE4 allele has been clearly associated with the presence of the beta amyloid in the brain of 853 β+ AD patients [37]. But the role of the E4 allele/alleles has not been elucidated in terms of poor survival or faster cognitive decline, even the results are contradictory according to different reports. In one of them, the presence of one/two APOE4 alleles was associated with faster cognitive decline in two prospective population-based cohorts following-up a total of 560 prevalent and incident AD cases [38]. However in a cohort of 99 AD patients the presence of at least one APOE4 allele was associated with a less aggressive progression of AD and decreased mortality (RR: 0,38; 95%CI: 0,17-0,84) in comparisons with non-APOE4 allele carriers [39] The same occurred in a cohort of 99 early onset AD [40].
▪ Neuropsychiatric symptoms
In Table 3 and Figure 2 are listed the prospective cohort studies focused on NPS and mortality in AD, with evidence level I. Considered together the high levels of NPS were predictive of mortality from moderate or severe stages of AD, according to a large prospective cohort of 1145 patients [11]. Separately analysed and taking into account the number of patients followedup, delusions were not clearly predictive of mortality. In three cohorts totalling 948 patients, delusions/psychosis increased the risk of mortality [41-43]. But in 5 cohorts totalling 1586 patients there was no statistical association between delusions/psychosis and death [23,44-47] Apathy was analysed in two cohorts; in one of them [43] the risk of death was significantly increased (HR: 3.22), but in the other one the HR did not reach statistical significance (HR: 1.22) [22]. For agitation/aggression, the risk of death was significant in two cohorts comprising 470 patients (HR: 2.98 and 1.94 respectively) [27,41] and not significant in one cohort including 179 patients (HR: 1.01). 45 Hallucinations were predictive of death in three cohorts [29,44,47] so was depression/affective disorder in other three cohorts [41,43,48].
| TYPE of SYMPTOM | Authors/year/n | Adjusted Relative Risk or HR and 95% CI |
|---|---|---|
| High/low level low of behavioral symptoms in BRSD | n=1145 [11] | Moderate stage to death: 3.33 Severe stage to death: 1.69 |
| Psychosis/delusions | n=456 [44] | 0,86 (0,65-1,13) |
| " | n=179 [45] | 0,93, NS |
| " | n=407 [29] | 1,22 (0,86-1,72) |
| " | n=335 [41] | 1,53, p=0.011* |
| " | n=99 [46] | NS |
| " | n=445 [47] | 1.34 (0.93-1.91) |
| " | n=491 [42] | 2.19 (1.13-4.22)* |
| “ | n=122 [43] | 3.61 ( 1.15-6.79 )* |
| Apathy | n=150 [22] | 1.24, NS |
| " | n=122 [43] | 3.22 ( 1.13-7.24 )* |
| Hallucinations | n=456 [44] | 1,49 (1,03-2,14)* |
| n=407 [29] | 1,7 (1.21-2.38)* | |
| n=407 [47] | 1.53 (0.92-2.54) | |
| n=135 [27] | 3.85 (1.35-11)* | |
| Agitation/aggression | n=179 [45] | 1,01 (0,78-1,34) |
| n=335 [41] | 1,94, p=0,004* | |
| n=135 [27] | 2.98 (1.35-6.61)* | |
| Depression/afective disorder | n=335 [41] | 1,51, p=0,003* |
| “ | n=1958 [48] | 1,88 (1,12-3,18)* |
| “ | n=122 [43] | 2.68 (0,96-6,37)* |
| Antipsychotic treatment | n=335 [41] | No influence |
| “ | n=779 [23] | 1.37 (1.01-1.85)* |
| “ | n=456 [44] | 1,26 (0.89-1.77) |
| “ | n=445 [29] | No influence |
| “ | n=179 [45] | 0,74; NS |
| “ | n=273 [59] | HR: 0.72 (0.30-0.86)* |
| “ | n=534 [57] | HR: 1.12 (0.59-2.12) |
| Sedatives/anxiolytics/hipnotics | [45] | 2,85 (1,6-3,5)* |
| Antidepressants | [45] | 0,85; NS |
| * means significant | ||
Table 3: Behavioral symptoms as Predictors of mortality in AD. Data from prospective cohorts.
▪ The risk of mortality in neuroleptics and other neurotropic drugs users
Atypical neuroleptics, and other sedative drugs are many times necessary to treat behavioural symptoms in patients with AD and other dementias, and are useful to treat aggression and psychosis according to a meta-analysis based on 16 placebo controlled trials, but with serious adverse events; mortality was not analysed here because of insufficient data [49]. A previous meta-analysis of 17 placebo controlled trials conducted by the FDA showed an increased mortality in the group on atypical neuroleptics, as a result of cardiovascular events; RR: 1.7; Furthermore, another meta-analysis of 12 trials and 11,463 patients did not confirm the increased risk of death in patients on either conventional or atypical neuroleptics (pooled RR 1,36; 95% CI: 0.83-2.24); only when three trials were excluded because of heterogeneity, then the RR was 2.08 (1.39-3.13) [50].
Data from many cohorts are available. With regard to retrospective cohorts and evidence level II, the results are consistent in some aspects and contradictory in others. From a retrospective population-based cohort in Italy comprising 4369 residents aged 60 or older it was concluded that mortality increased two fold with atypical neuroleptics and 5 fold with conventional neuroleptics in comparison with non-treated patients [51]. In a nationwide Danish registry with 45,894 followed-up for 10 years showed that cumulative antipsychotic dosage increased mortality rates from cardiovascular events and infections between 1.92 and 2,31 times [52]. Two large retrospective studies comprising respectively 90,786, and 43,183 communitydwelling demented patients aged 65 or older, found a dose-dependent increased mortality in treated patients, with the highest rates in those treated with either haloperidol or risperidone, and the lowest in the group on quetiapine [53,54]. However not all retrospective studies point in the same direction. In a retrospective cohort of nursing home AD patients, 3696 with behavioural symptoms were treated respectively with either antipsychotics (2035 patients) or antidepressants (1661 patients) for 3 consecutive months; mortality rates were lower in both treated groups than in non-treated group [55]. In a meta-analysis focused on risperidone trials, cardiovascular events and all cause mortality were more frequent in the treated group but without statistical significance (evidence level I) [56]. Moreover, in a large retrospective cohort including 3 sub-cohorts of nursing home Medicare beneficiaries (severe mental illness: 5621 patients; dementia with behavioural symptoms: 1090 patients, and 2100 patients with delirium), the mortality rates were lower in the groups of patients treated with antipsychotics; RR: 0,52 (0,36-0,76) [57].
On the light of prospective cohort studies of Table 3 (evidence level I), the trends are more consistent. Only one study of 779 patients with dementia in 7, yielded an increased mortality with neuroleptics [23] versus the 2756 followedup in the other 6 cohorts [29,41,44,45,58]. Only one prospective cohort of 273 patients was nursing home-based, and the mortality rate was even higher in the group of non-treated patients than in those treated with neuroleptics, but the follow-up period, just one year, was shorter than the rest of the cohorts [59].
Interestingly, the use of any: sedatives, anxiolytics, or hypnotics, as a separate variable, but not antidepressants. increased the risk of death more than twice (HR: 2.85; 95% CI: 1.6- 3.5) [45]. In return the use of antidepressants in nursing home patients with AD increased the risk of fractures in comparison with antipsychotics, adjusted HR: 1.35 (1.10-1.66); considering the variable risk of falls or fractures the HR remained significant: 1.16 (1.02-1.32) [60].
▪ Predictors of faster cognitive decline
In Table 2 are listed the possible predictive variables of faster cognitive decline, and in Table 4 the prospective cohorts analysing the risk of faster progression in AD and related dementias. Having a look at the Table 4, it can be observed that most studies point towards an increased risk of faster cognitive deterioration in patients having high levels of behavioural symptoms [11,61,62] psychosis/delusions [26,30,41,44,45,47,63,64], hallucinations [27,44], agitation/aggression [27,41,64] and the use of antipsychotics. In two cohorts including 695 patients [45,47] antipsychotics use predicted faster deterioration versus one cohort including 185 patients that did not [65]. A longitudinal study of 71 demented patients showed that the rate of cognitive decline in conventional neuroleptic users was twice that the observed in non-users [66]. Depression did not predict faster cognitive decline in three cohorts [41,45,64].
| TYPE of symptom | Study | Adjusted RR or HR, and 95%CI | |
|---|---|---|---|
| Psychosis/hallucinations/agitation/aggression/sleep disturbance | n=65 [61] | HR not reported. Regression coefficients: p<0.01;< 0.05; <0.01; <0.005; <0.005; respectively | |
| High vs low level low of behavioral symptoms in BRSD | n= 1145 [11] | From mild to moderate stage. HR: 1.33; p=0.09 |
|
| High vs low level of NPS |
43 n=122 NPI Scores by clusters |
2.31 (0.96-5.55) | |
| Psychosis/delusions | [45] | 2.02 (1.5-3.13)* | |
| "" | [41] | 2.21 (1.17-4.17)* | |
| [28] | 1.85 (1.18-2.90)* | ||
| n=456 | 1.5 (1.07-2.08)* | ||
| n=445 [47] | 2.55 (1.48-5.53)* | ||
| n=65 [26 ] | 1.25 (0.88-1.78) | ||
| n=517 [64] | P<0.001, in survival curve comparison. HR not reported | ||
| n=521 [30] | 2.06 (1.22-3.48)* | ||
| Hallucinations | n=135 [27] | 3.85 (1.35-11)* | |
| n=456 [44] | 1.62 (1.06-2.47)* | ||
| Antipsychotic treatment | n=179 [45] | 2.02 ( (1.3-2.3)* | |
| n=184 [65] | NS | ||
| n=445 [47] | 1.6 (1.03-2.49)* | ||
| n=71 [66] | P=0.02. HR not reported | ||
| Depression | [41] | 1.21 (0,69-2.14) NS | |
| " | [5] | 0.78 (0.57-1.07) NS | |
| n=517 [64] | NS in curve comparison, | ||
| Agitation/aggression | [41] | 2.88 (1.39-5.96)* | |
| n=135 [27] | 2.98 (1.35-6.65)* | ||
| n=517 [64] | P<0.00 | ||
Table 4: Predictors of faster cognitive decline in AD.Data from prospective cohorts.
Other little studied predictors of faster decline have been claimed such as early language and executive function impairment [16,67,68] high levels of CSF tau and p-tau levels [69] low plasma levels of Abeta40 and Abeta42 and elevated plasma levels of high sensitivity C-reactive protein [70] impairment of daily living activities [71], caregivers attitude [72], baseline severity of dementia [23,62,64,73] nutritional status [30,74] and cortical atrophy on MR-based volumetry [75].
▪ Cholinesterase inhibitor use and outcomes
The effect of ChEI use on survival has not been clearly elucidated, but the trend is for increased survival time. The treatment with cholinesterase inhibitors (higher doses and longer duration of treatment) was associated with longer survival and slower cognitive deterioration in a prospective multi-centre study of ChEI in the clinical practice comprising 1021 communitybased AD patients followed-up for up to 16 years [76]. Conversely, in a sub-cohort of 220 nursing home-based patients from the same multi-centre study, the use of ChEI did not alter survival time [77]. Positive results were also seen in a large database-linked retrospective cohort of 2646 patients (evidence level II), with lower mortality rates in ChEI users, adjusted HR: 0.77 (0.67- 0.87) [78]. Surprisingly, in a cohort of 156 AD patients, those who were taking cholinesterase inhibitors experienced a worse functional and cognitive progression compared with ChEInaïve patients [79].
Discussion
In this systematic review we have taken into account a large number of patients and cohorts dealing with predictors of mortality and faster cognitive decline in AD. From prospective cohorts we found enough evidence that NPS increased the risk of mortality and faster cognitive decline in AD patients.
An increased risk of mortality attributed to the use of neuroleptics was found in meta-analysis of clinical trials and in some retrospective cohorts but not in the prospective ones. Although prospective cohorts are a better quality source of information, the number of patients followedup is much lower than in the retrospective cohorts: 3808 versus 211,908 patients. However the data are more imprecise and less reliable in retrospective studies. Therefore, there is not a clear evidence on whether the risk of neuroleptics outweigh the benefits in patients with dementia and behavioural symptoms not controlled by other means.
The clinical trials of antipsychotics in dementia use to be conducted for short-term periods (6- 12 weeks), whereas the cohorts are followedup for years. The discrepancies found in the retrospective cohorts might be explained by the different setting in which the patients live. Moreover, it can be argued that the results from observational studies of nursing home patients cannot be extrapolated to communitydwelling ones, as the nursing home-dwelling patients present higher degrees of cognitive and functional impairment. Therefore it is likely that the benefits of neuroleptics outweigh the risks in the subpopulation of patients living in nursing homes, and in the community-dwelling patients with highly disturbing NPS non-controlled by other means.
Switching from atypical to conventional neuroleptics is not of help as the risk of mortality was higher in the elderly treated with conventional neuroleptics than in those treated with the atypical ones in two large retrospective community-based cohorts including 43,183 and 22,890 respectively [54,80]. With regard to other pharmacologic treatments, eg: hypnotics, anxiolytics and antidepressants, they are not free of serious side-effects as it was commented before. Well known is the QT space prolongation associated with the use of citalopram which is given for agitation.
It is also interesting the clinical progression of AD across the different parts of Europe [81]. Functional decline was faster in Southern Europe but caregiver burden increased more in Northern Europe. The rate of admissions to nursing home was lowest in Southern Europe than in the North. It may reflect the better-off economic status in Northern Europe than in the South, given that the access to nursing home and other services are less affordable in the South of Europe. Living alone and physical limitations are frequent causes of choosing a nursing home to stay instead of household, and it does not mean worse cognitive function or having burdensome NPS. For this reasons it is of questionable value to use the nursing home admission as an indicator of worse outcome in AD patients, and as an end-point in clinical trials. There were no differences in the progression of cognitive and behavioural scales across the regions. While behavioural symptoms progressed linearly, the cognitive decline was not linear [81].
Many can wonder about the reason of the great differences in the outcomes of AD patients. There is no clear response but the field of proteomics might offer a glimpse of hope for a better understanding of the neurobiological basis of symptoms and progression. In this respect a combination of the levels of CSF biomarkers (Aβ42, p-tau, and ubiquitin, ApoE genotype and clinical onset resulted in five subgroups or clusters with different clinical profile and a possible different response to drugs [82]. The mechanisms by which neuroleptics have a negative impact in AD may be the accelerated development of neurofibrillary tangles in the brain cortex, as it was demonstrated in a study with 102 schizophrenics; the density of tangles was twice in treated patients (74%) as those nontreated (36%) [83]. Other possible explanation is the dysregulation of tau phosphorylation through the inhibition of protein phosphatase- 2B, involved in the dephosphorylation process, caused by clssic neuroleptics such as clorpromazine, clozapine, and trifluoperazine [83].
Strengths and Limitations
The strengths of this systematic review are: the use of data of patients from prospective cohorts with clear criteria of patient inclusion, the additional use of large population-based retrospective cohorts and nursing home-based cohorts, as well, so as to better reflect the real world effects of drugs. The work has been done free of commercial bias, and only based on objective data. The limitations can be listed as follows: the duration of the follow-up of the cohorts was variable, some of them included prevalent AD cases, others incident cases, and others both prevalent and incident cases. The patients included not always were in the same stage of the disease. Meta-analysis and metaregression have not been possible because of the incomplete datasets in some studies
In conclusion, from the data of this systematic review there is enough evidence supporting the association between high levels of neuropsychiatric symptoms and mortality, and faster cognitive deterioration (level I of evidence). Individually considered, the presence of hallucinations, agitation/aggression or depression, at baseline or in the follow-up, is predictive of poor outcomes (level I of evidence). Unlike clinical trials, prospective cohort studies do not support the hypothesis that neuroleptics increase mortality in AD and related dementias (level I of evidence). From large retrospective cohorts, we can conclude that conventional neuroleptics are most likely, or at least as likely, to be associated with a risk of death as the atypical ones (evidence level II). However the use of neuroleptics is associated with faster cognitive decline (evidence level I). The use of antidepressants does not seem to cause higher mortality or poorer cognitive outcomes but increase the risk of falls and fractures, and may have also negative influence on sleep and behaviour (evidence level I). As usual, a judicious use of psychotropic drugs is advised, bearing in mind the ups and downs in every patient.
Conflict of Interest
We declare no competing interests.
References
- Zanetti O, Solerte SB, Cantoni F. Life expectancy in Alzheimer's disease. Arch. Gerontol. Geriatr 49(1), 237-243 (2009).
- Beydoun MA, Beydoun HA, Garnaldo AA, et al. Nationwide inpatient prevalence, predictors and outcomes of Alzheimer's disease among older adults in the United States, 2002-2012. J. Alzheimers. Dis 48(2), 368-375 (2015).
- Li XL, Hu N, Tan MS, et al. Behavioral and psychological symptoms in Alzheimer's disease. Biomed. Res. Int 10(6), 511-517 (2014).
- Wilkosz PA, Miyahara S, Lopez OL, et al. Prediction of psychosis onset in Alzheimer disease: The role of cognitive impairment, depressive symptoms, and further evidence for psychosis subtypes. Am. J. Geriatr. Psychiatry 14(1), 352-360 (2006).
- D'Onofrio G, Panza F, Sancarlo D, et al. Delusions in patients with Alzheimer's disease. A multidimensional approach. J. Alzheimers. Dis 51(2), 427-437 (2016).
- Modrego PJ. Depression in Alzheimer's disease. Pathophysiology, diagnosis, and treatment. J. Alzheimers. Dis 21(4), 1077-1087 (2010).
- Khundakar AA, Thomas AJ. Neuropathology of depression in Alzheimer's disease: current knowledge and the potential for new treatments. J. Alzheimers. Dis 44(1), 27-41 (2015).
- Rodrigues R, Petersen RB, Perry G. Parallels between major depressive disorder and Alzheimer's disease: role of oxidative stress and genetic vulnerability. Cell. Mol. Neurobiol 34(1), 925-949 (2014).
- Teri L, Ferretti LE, Gibbons LE, et al. Anxiety of Alzheimer's disease: prevalence, and comorbidity. J. Gerontol. A. Biol. Sci. Med. Sci 54(7), 348-352 (1999).
- Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: Systematic review and meta-analysis. J. Affect. Disord 190(1), 264-271 (2016).
- Neumann PJ, Araki SS, Arcelus A, et al. Measuring Alzheimer's disease progression with transition probabilities: estimates from CERAD. Neurology 57(6), 957-964 (2001).
- Ballard CG, Gauthier S, Cummings JL, et al. Management of agitation and aggression associated with Alzheimer's disease. Nat. Rev. Neurol 5(5), 245-255 (2009).
- Paulsen JS, Salmon DP, Thai LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology 54(10), 1965-1971 (2000).
- Cummings J, Mintzer J, Brodaty H, et al. Agitation in cognitive disorders: International Psychogeriatrics Association provisional consensus clinical and research definition. Int. Psychogeriatr 27(1), 7-17 (2015).
- Lapane KL, Gambassi G, Landi F, et al. Gender differences in predictors of mortality in nursing home residents with AD. Neurology 56(5), 650-654 (2001).
- Clauss JJ, Walstra GJ, Bossuyt PM, et al. A simple test of copying ability and sex define survival in patients with early Alzheimer's disease. Psychol. Med 29(2), 485-489 (1999).
- Bowen JD, Malter AD, Sheppard L, et al. Predictors of mortality in patients diagnosed with porbable Alzheimer's disease. Neurology 47(2), 433-439 (1996).
- Russ TC, Batty GD, Starr JM. Cognitive and behavioural predictors in Alzheimer's disease: results from a sample of treated patients in a tertiary-referral memory clinic. Int. J. Geriatr. Psychiatry 27(8), 844-853 (2012).
- Rountree SD, Chan W, Pablik VN, et al. Factors that influence survival in a probable Alzheimer's disease cohort. Alzheimer. Res. Ther 4(3), 16 (2012).
- Moritz DJ, Fox PJ, Luscombe FA, et al. Neurological and psychiatric predictors of mortality in patients with Alzheimer disease in California. Arch. Neurol 54(7), 878-885 (1997).
- Gambassi G, Laplane KL, Landi F, et al. Gender differences in the relation between comorbidity and mortality of patients with Alzheimer's disease. Systematic assessment of Geriatric drug use via epidemiology (SAGE) study group. Neurology 53(3), 508-516 (1999).
- Spalletta G, Long JD, Robinso RG, et al. Longitudinal neuropsychiatric predictors of death in Alzheimer's disease. J. Alzheimers. Dis 48(3), 627-636 (2015).
- Connors MH, Ames D, Boundy K, et al. Predictors of mortality in dementia: The PRIME study. J. Alzheimers. Dis 52(3), 967-974 (2016).
- Stern Y, Mayeux R, sano M, et al. Predictors of disease course in patients with probable Alzheimer's disease. Neurology 37(10), 1649-1653 (1987).
- Chui HC, Lyness SA, Sobel E, et al. Extrapyramidal signs and psychiatric symptoms predict faster cognitive decline in Alzheimer's disease. Arch. Neurol 51(7), 676-681 (1994).
- Stern Y, Albert M, Brandt J, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer's disease: prospective analysis from the predictors study. Neurology 44(12), 2300-2307 (1994).
- Wilson RS, Krueger KR, Kamenetsky JM, et al. Hallucinations and mortality in Alzheimer disease. Am. J. Geriatr. Psychiatry 13(11), 984-990 (2005).
- Tchalla AE, Clement JP, Saulnier I, et al. Predictors of rapid cognitive decline in patients with mild-to-moderate Alzheimer disease: A prospective cohort study with 12-month follow-up performed in in memory clinics. Dement. Geriatr. Cogn. Disord 45(1), 1-10 (2018).
- Rasmusson DX, Carson KA, Brookmeyer R, et al. Predicting rate of cognitive decline in probable Alzheimer's disease. Brain. Cogn 31(2), 133-147 (1996).
- Musicco M, Palmer K, Salamone G, et al. Predictors of progression of cognitive decline in Alzheimer's disease: the role of vascular and sociodemographic factors. J. Neurol 256(8), 1288-1295 (2009).
- Sakurai H, Hanyu H, Sato K, et al. Vascular risk factors and progression in Alzheimer's disease. Geriatr. Gerontol. Int 11(2), 211-214 (2011).
- Quiao J, Lu WH, Wang J, et al. Vascular risk factors aggravate the progression of Alzheimer's disease: a 3-year-follow-up study of Chinese population. Am. J. Alzheimers. Dis 29(6), 521-525 (2014).
- Helzner EP, Luchsinger JA, Scarmeas N, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch. Neurol 66(3), 343-348 (2009).
- Kume K, Hanyu H, Sato T, et al. Vascular risk factors are associated with faster decline of Alzheimer disease: a longitudinal SPECT study. J. Neurol 258(7), 1295-1303 (2011).
- Mattsson N, Groot C, Jansen WJ, et al. Prevalence of the apolipoprotein E ɛ4 allele in amyloid ß positive subjects across the spectrum of Alzheimer's disease. Alzheimers. Dement 18(1), S1552-5260 (2018).
- Consentino S, Scarmeas N, Helzner E, et al. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology 70(2), 1842-1849 (2008).
- Stern Y, Brandt J, Albert M, et al. The absence of an apolipoprotein epsilon4 allele is associated with a more aggressive form of Alzheimer's disease. Neurology 41(5), 615-620 (1997).
- van der Vlies AE, Koedam EL, Pinenburg YA, et al. Most rapid cognitive decline in AOPE epsilon4 negative Alzheimer's disease with early onset. Psychol. Med 39(11), 1907-1911 (2009).
- Peters ME, Schwartz S, Han D, et al. Neuropsychiatric symptoms as predictors of progression to severe Alzheimer's dementia and death: the Cache County Dementia Progression Study. Am. J. Psychiatry 172(5), 460-465
- Vilalta-Franch J, Lopez Pousa S, Calvo-Perxas L, et al. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am. J. Geriatr. Psychiatry 21(11), 1135-1143 (2013).
- Tun SM, Murman DL, Long HL, et al. Predictive validity of neuropsychiatric subgroups on nursing home placement and survival in patients with Alzheimer disease. J. Am. Geriatr. Psychiatry 15(4), 314-327 (2007).
- Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch. Neurol 62(10), 1601-1608 (2005).
- Lopez OL, Wisniewski SR, Becker JT, et al. Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer disease. Arch. Neurol 56(10), 1265-1272 (1999).
- Magni E, Binetti G, Bianchetti A, et al. Risk of mortality and institutionalization in demented patients with delusions. J. Geriatr. Psychiatry. Neurol 9(3), 123-126 (1996).
- Connors MH, Ames D, Woodward M, et al. Psychosis and clinical outcomes in Alzheimer disease: A longitudinal study. Am. J. Geriatr. Psychiatry 26(3), 304-313 (2018).
- Lara E, Haro JM, Tang MX, et al. Exploring the excess mortality due to depressive symptoms in a community-based sample: The role of Alzheimer's disease. J. Affect. Disord 202(1), 163-170 (2016).
- Ballard C, Walte J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease. Cochrane. Database. Syst. Rev 1(2), CD003476 (2006).
- Zhai I, Yin S, Zhang D. Association between antipsychotic drugs and mortality in older persons with Alzheimer's disease: A systematic review and meta-analysis. J. Alzheimers. Dis 52(2), 631-639 (2016).
- Musicco M, Palmer K, Russo A, et al. Association between prescription of conventional or atypical antipsychotic drugs and mortality in older persons with Alzheimer's disease. Dement. Geriatr. Cogn. Disord 31(3), 218-224 (2011).
- Nielsen RE, Lolk A, Rodrigo-Domingo M, et al. Antipsychotic treatment effects on cardiovascular, cancer, infection, and intentional self-harm as cause of death in patients with Alzheimer's dementia. Eur. Psychiatry 42(1); 14-23 (2017).
- Maust DT, Kim HM, Sevfried LS, et al. Antipsychotics, other neurotrpics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry 72(5), 438-445 (2015).
- Gerhard T, Huybrechts K, Olfson M, et al. Comparative mortality risks of antipsychotic medications in community dwelling older adults. Br. J. Psychiatry 205(1), 44-51 (2014).
- Tosto G, Gasparini M, Brickman AM, et al. Neuropsychological predictors of rapidly progressive Alzheimer's disease. Acta. Neurol. Scand 132(6), 417-422 (2015).
- Huang TY, Wei YJ, Moyo P, et al. Treated behavioral symptoms and mortality in Medicare beneficiaries in nursing homes with Alzheimer's disease and related dementia. J. Am. Geriatr. Soc 63(9), 1757-1765 (2015).
- Katz I, de Deyn PP, Mintzer J, et al. The efficacy and safety of risperidone in the treatment of psychosis of Alzheimer's disease and mixed dementia: a meta-analysis of 4 placebo-controlled clinical trials. Int. J. Geriatr. Psychiatry 22(5), 475-484 (2007).
- Simoni-Wastilla L, Wei YJ, Lucas JA, et al. Mortality risk of antipsychotic dose and duration in nursing home residents with chronic or acute indications. J. Am. Geriatr. Soc 64(5), 973-980 (2016).
- Gardette V, Lapeyra-Maestre M, Coley N, et al. Antipsychotic use and mortality risk in community-dwelling Alzheimer's disease patients: evidence for a role of dementia severity. Curr. Alzheimer. Res 9(9), 1106-1116 (2012).
- Suh GH, Shah A. Effects of antipsychotics on mortality in elderly patients with dementia: a 1-year prospective study in a nursing home. Int. Psychogeriatr 17(3), 429-441 (2005).
- Wei YJ, Simoni-Wastilla L, Lucas JA, et al. Fall and fracture risk in nursing home residents with moderate-to-severe behavioral symptoms of Alzheimer's disease and related dementias initiating antidepressants or antipsychotics. J. Gerontol. Biol. Sci. Med. Sci 72(5), 695-702 (2017).
- Mortimer JA, Ebbitt B, Jun SP, et al. Predictors of cognitive and functional progression in patients with probable Alzheimer's disease. Neurology 42(9), 1689-1696 (1992).
- Tun SM, Murman DL, Long HL, et al. Predictive validity of neuropsychiatric subgroups on nursing home placement and survival in patients with Alzheimer disease. Am. J. Geriatr. Psychiatry 15(4), 314-327 (2007).
- Stern RG, Mohs RC, Davidson M, et al. A longitudinal study of Alzheimer's disease: measurement, rate, and predictors of cognitive deterioration. Am. J. Psychiatry 151(1), 390-396 (1994).
- Zahodne LB, Ornstein K, Cosentino S, et al. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am. J. Geriatr. Psychiatry 23(2), 130-140 (2015).
- LIvingston G, Walker AE, Katona CLE, et al. Antipsychotics and cognitive decline in Alzheimer's disease: the LASER-Alzheimer's longitudinal study. J. Neurol. Neurosurg. Psychiatry 78(1), 25-29 (2007).
- McShane R, Keene J, Gedling K, et al. Do neuroleptic drugs hasten cognitive decline in dementia? Prospective study with necropsy follow-up. BMJ 314(7076), 266-270 (1997).
- Musicco M, Salamone G, Caltagirone C, et al. Neuropsychological predictors of rapidly progressing patients with Alzheimer's disease. Dement. Geriatr. Cogn. Disord 30(3), 219-228 (2010).
- Tosto G, Gasparini M, Brickman AM, et al. Neuropsychological predictors of rapidly progressive Alzheimer's disease. Acta. Neurol. Scand 132(6), 417-422 (2015).
- Degerman Gunnarsson M, Lannfelt L, Ingelsson M, et al. High tau levels in cerebrospinal fluid predict rapid decline and increased dementia mortality in Alzheimer's disease. Dement. Geriatr. Cogn. Disord 37(3), 196-206 (2014).
- Locascio JJ, Fukumoto H, Yap L, et al. Plasma amyloid beta-protein and C-reactive protein in relation to the rate of progression of Alzheimer disease. Arch. Neurol 65(6), 776-785 (2008).
- Liang FW, Chan W, Chen PJ, et al. Cognitively-related basic activities of daily living impairment greatly increases the risk of death in Alzheimer's disease. PLoS One 11(8), e0160671 (2016).
- McClendon MJ, Smyth K, Neundorfer MM. Survival of persons with Alzheimer's disease: caregiver coping matters. Gerontologist 44(4), 508-519 (2004).
- Ruitenberg A, Kalmijn S, de Ridder MA, et al. Prognosis of Alzheimer's disease. The Rotterdam Study. Neuroepidemiology 20(3), 188-195 (2001).
- Dumont C, Voisin Tnourhashemi F, Andrieu S, et al. Predictive factors for rapid loss on the mini-mental state examination in Alzheimer's disease. J. Nutr. Health. Aging 9(1), 163-116 (2005).
- Brickman AM, Honig LS, Scarmeas N, et al. Measuring cerebral atropphy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer's disease. Arch. Neurol 65(9), 1202-1208 (2008).
- Wattmo C, Londos E, Minthon L. Longitudinal associations between survival in Alzheimer's disease and cholinesterase inhibitor use, progression, and community-based services. Dement. Geriatr. Cogn. Disord 40(6), 297-310 (2015).
- Wattmo C, Londos E, Minthon L. Cholinesterase inhibitors do not alter the length of stay in nursing homes among patients with Alzheimer's disease: a prospective, observational study of factors affecting survival time from admission to death. BMC Neurol 16(1), 156 (2016).
- Mueller C, Perera G. Hayes RD, et al. Associations of acetylcholinestarase inhibitor treatment with reduced mortality in Alzheimer's disease: a retrospective survival analysis. Age. Ageing 47(1), 88-94 (2018).
- Sona A, Zhang P, Ames D, et al. Predictors of rapid cognitive decline in Alzheimer's disease: results from the Australian Imaging, biomarkers and lifestyle (AIBL) study of ageing. Int. Psychogeriatr 24(2), 197-204 (2012).
- Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N. Engl. J. Med 353(22), 2335-2341 (2005).
- Vellas B, Hausner L, Frölich L, et al. Progression of Alzheimer disease in Europe: data from the European ICTUS Study. Curr. Alzheimer. Res 9(8), 902-912 (2012).
- Iqbal K, Flory M, Khatoon S, et al. Subgroups of Alzheimer's disease based on cerebrospinal fluid molecular markers. Ann. Neurol 58(5), 748-752 (2005).
- Wisniewski HM, Constantidinis J, Wegiel J, et al. Neurofibrillary pathology in brains of elderly schizophrenics treaated with neuroleptics. Alzheimer. Dis. Assoc. Disord 8(4), 211-227 (1994).
- Gong CX, Shaikh S, Grundke-Iqbal I, et al. Inhibition of protein phosphatase-2B (calcineurin) activity towards Alzheimer abnormaly phosphorylated tau by neuroleptics. Brain. Res 741(1), 95-102 (1996).