Review Article - (2019) Volume 9, Issue 6
Association of SLC6A4 5-HTTLPR and 5HTR2A T102C in the Neurobiological Domains Associated with Time Perception: Genetic and Behavioral Correlates
- Corresponding Author:
- Victor Marinho
Federal University of Piauí, Brazil. Neuro-innovation Technology & Brain Mapping Laboratory-Av
São Sebastião nº 2819–Nossa Sra. de Fátima–Parnaíba
PI, CEP: 64202-020, Brazil
Tel: +55 86 994178117 E-mail: [email protected]
Abstract
Research at the molecular level aims to integrate neurobiological information in a more detailed manner on behavioral phenotypes associated with the judgment of time intervals. The genetic polymorphisms SLC6A4 5-HTTLPR and 5HTR2A T102C as modulators of serotoninergic levels are associated with several neurobiological aspects inbuilt in sub-second and suprasecond timing. In this sense, a state-of-the-art review was performed with 60 studies, among them experimental and reviews, to synthesize the main findings on the action of genetic bases of the serotoninergic system in timing. The findings indicate that the level of expression of serotonin receptors and transporters change neural inputs in neurobiological domains related to the time perception.
Keywords
Time perception, Genetic, Serotonin, SLC6A4 5-HTTLPR, 5HTR2A T102C, Neurobiological aspects
Introduction
Time and space are fundamental dimensions in the existence of species and crucial for the synchronization of daily activities such as walking, talking, playing and practicing sports, besides acting in neurobiological domains (i.e., executive functions, cognition and circadian regulation [1]. The timing involved in these activities is mediated by domains of time intervals ranging from milliseconds to hours of the day [1,2], which is related to the neurochemistry of the Central Nervous System (CNS) [2-5]. Thus, the CNS through the network and neurotransmission loops identify the process and interpret events in a dynamic system, linked to interactions granted by experiences and conditions provided by the environment. This capacity of timing and temporal synchronism is partly mediated by serotoninergic neurochemistry, which enables adjuvant action in the coding of different extrinsic and/or intrinsic stimuli [3,5]. Therefore, the perceptual capacity of time requires a complex mechanism of neural inputs and outputs that can be changed by the state of serotonin concentration and other neurotransmitters [6-8].
Without the action of neurotransmitters, in focus on serotoninergic mediation, cognitive functions, especially actions of decision making, learning, and visual awareness would be severely impaired. Thus, the timing synchronization of serotoninergic modulation was proposed as essential for basic learning processes and environmental stimuli coding [9-11]. That is, it detects the number of stimuli per time interval mediated by neurotransmission [12,13], which according to the Scalar Expectation Theory (SET) is interesting from the neurophysiological and neurochemical point of view, since it is composed of three processing levels that potentially correspond to functional neuroanatomy related to dopaminergic and serotonergic pathways. Based on SET, an intrinsic “pacemaker” mechanism produces a series of pulses that are “collected”. These pulses are subsequently “counted” to estimate durations. Neurophysiologically, a comparison system connects the output of the time counting process with memorized time references in order to estimate the duration of the event [12,14]. This synchronization process is reinforced by neurochemical mediations, and changes in the level of neurotransmitters can impair temporal processing [9,13].
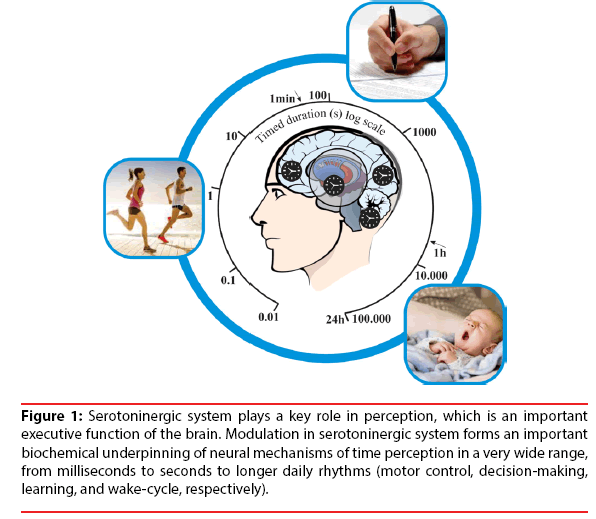
In this case, it was observed that serotoninergic action has a strong participation in neurofunctional modulation, daily rhythm patterns and sleep patterns, both essential in neurobiological functions incorporated into the time perception (Figure 1) [6,7,15,16].
Figure 1:Serotoninergic system plays a key role in perception, which is an important executive function of the brain. Modulation in serotoninergic system forms an important biochemical underpinning of neural mechanisms of time perception in a very wide range, from milliseconds to seconds to longer daily rhythms (motor control, decision-making, learning, and wake-cycle, respectively).
Some substances modify the serotoninergic concentration (for example, 8-Hydroxy-DPAT hydrobromide (8-OH-DPAT) and fenfluramine) [17,18], and thus interfere in the processing of temporal information. This was observed in the study of Lake and Meck [19], with the injection of agonist drugs and dopaminergic and serotoninergic antagonists in the striated nucleus of rats, therefore, they observed a considerable displacement of the time interval judgment [19,20]. The approach is exemplified by Di Giovanni et al. [21], who observed interactions between dopamine and serotonin in the brain through electrophysiological data in vivo and in vitro. The results indicated that dopaminergic neurons receive serotoninergic prominences, originating from brainstem based cores. Therefore, the brain chemical modulation by means of serotonergic relationships has an impact on the perception of time based on functional changes in the serotonin carrier and type 2A receptors due to phosphorylation, protein-protein interactions and changes in intracellular serotoninergic location [5,22].
In a current context, studies relate the genetic influence on neurotransmission during time perception (Table 1). The study by Meck et al. [20] demonstrated the genetic architecture involved in timing through magnetic resonance imaging in knocked out rats for the SLC6A3 and HTR2A genes. The results showed less activation in prefrontal cortex (PFC) neural circuits and subcortical regions during the encoding of stimuli. Therefore, changes in the genes expressions that regulate levels of neurotransmitters in the striated body and in other areas acting as intrinsic clocks of time perception may allow variations in the speed of the “internal clock” and the sleep-wake cycle predominantly mediated by changes in dopaminergic and serotonergic receptors and transporters [9,23].
| Reference | Genotyping | Sample and Protocols | Stimulus | Target-Interval | Synthesis of Results | |
|---|---|---|---|---|---|---|
| [13] | SLC6A3 3'-UTR VNTR SLC6A3 intron 8 VNTR |
Sample: 108 healthy male individuals. Cognitive tasks: Time estimation task. | Visual | 1 s, 4 s, 7 s, 9 s |
The intron 8 VNTR polymorphism modulates subjective timekeeping for the 1 s and 9 s intervals and the combinatorial effect of the polymorphisms only in 1 s, which alters the internal clock speed and decreases the accuracy of temporal judgment. | |
| [30] | COMT Val158Met | Sample: 66 healthy individuals. Cognitive evaluation: STPI, AUDIT, DASS, OCDS, and BIS. Administration of 200 mg tolcapone or placebo in a randomized. Cognitive task: Time estimation associated with fMRI image. | Visual | 2 s and 5 s | The COMT inhibitor tolcapone (COMT Val158) reduces impulsive decision-making in a delay discounting paradigm. Consistent with the hypothesized role of dopamine in component processes related to impulsivity and brain circuitry. The result demonstrated that time perspective can be ameliorated by a single dose of the COMT inhibitor tolcapone and that these effects cannot be explained by session effects or changes in low-level motor responding. | |
| [4] | SLC6A4 5-HTTLPR | Sample: 273 young healthy. Cognitive tasks: (memory and Face Identity Perception tasks). | Visual Face images |
Seconds range, varied for each participant. | There was neither an association between the 5HTTLPR genotype and cognitive tasks, but there might be a tendency for better performance of SL as compared with SS carriers for fEP. | |
| [16] | SLC6A4 5-HTTLPR, 5HT2A T102C, DRD2/ANKK1-Taq1A, SLC6A3 3'-UTR VNTR, COMT Val158Met, MAOA VNTR, and CLOCK genes. | Sample: 647 healthy individuals, Questionnaire. Cognitive test. Production and Discrimation tasks. | Visual Auditory |
1 s, 3 s, 6 s, 12 s, 15 s | Stability of an individual’s temporal accuracy and precision across in supra-second intervals (ranging from 3s to 15s) in the cognitive tasks and time perception tasks. Female participants are more likely to underestimate time production task when an explicit counting strategy is not employed | |
| [34] | COMT Val158Met, 5HTR2A T102C. | Sample: 90 healthy Japanese. Cognitive tasks and fMRI study. | Visual | 2 s, 3 s | Results demonstrate that the COMT genotypes are related to recognition accuracy, whereas the 5HTR2A genotypes are associated with RTs for recognition. In addition, strong connectivity in the cingulo-frontal networks is closely linked to a better working memory performance, regardless of the genotypes. | |
| [3] | COMT Val158Met, SLC6A3 3′UTR VNTR and DRD4 exon 3 VNTR. | Sample: 52 healthy Estonians. Cognitive tasks and Discrimination task. |
Visual | 23 ms, 70 ms, 105 ms, 735 ms, | SLC6A3 variability no-showed difference in the study. COMT Val158Met and DRD4 exon 3 VNTR differ in their effects on attentional functions as explicated in long-SOA metacontrast. | |
| [28] | DRD2/ANKK1-Taq1A. | Sample: 25 healthy individuals. Temporal or color Discrimination task and fMRI acquisition | Visual | 350 ms, 400 ms, 450 ms, 550 ms, 600ms, 650 ms; 1.4 s, 1.8 s, 2 s, 2.2 s, 2.4 s, 2.6 s. | A1 carriers of the Taq1A polymorphism exhibited worse performance on a temporal task. However, greater activation in the striatum and right dorsolateral prefrontal cortex, as well as reduced volume in the cerebellar. | |
| [33] | DRD2/ANKK1-Taq1Aand COMT Val158Met. | Sample: 41 healthy individuals. Time perception tasks. Fixed-intervals tasks. | Visual | 10 s, 17 s. | DRD2/ANKK1-Taq1A in the striatum and COMT Val158Met, affecting the breakdown of dopamine in the prefrontal cortex— to interval timing and reward magnitude modulation of decision thresholds. | |
| [37] | COMT Val158Met, SLC6A3 3’-UTR VNTR. | Sample: 95 healthy individuals. 64-channel EEG study. Continuous performance test. | Visual | 450 ms, 600 ms, 750 ms, 900 ms. | Effects of SLC6A3 and COMT on the occipitotemporal activity in CNV. In addition, there was a trend towards an interaction between the two polymorphisms. | |
| [20] | SLC6A3 +/+ rats SLC6A3 -/- rats SLC6A3 +/- rats |
Sample: DAT-mutant rat. Peak interval task. Administration: Methamphetamine hydrochloride. | Visual | 15 s, 20 s, 45 s, 140 s, 200 s. | Complete loss of temporal control and altered sensitivity to drugs. Lower threshold for initiating responding in the timing task. | |
| [29] | DRD2/ANKK1-Taq1A and COMT Val158Met. | Sample: 65 healthy individuals. Temporal discrimination task and motor tempo task. | Visual | 500 ms, 2 s | DRD2/ANKK1-Taq1A (A1+ allele) was associated with variability for the 500ms duration only, whereas the COMT Val158Met (Val/Val) was associated with variability for the 2000ms duration only. Additionally, the DRD2/ANKK1-Taq1A was associated with slower preferred motor time. | |
| [32] | SLC6A3 +/+ rats SLC6A3 -/- rats SLC6A3 +/- rats |
Sample: KD rat and WT rat. Peak interval task and administration of the Raclopride. | Visual | 20 s, 40 s, 60 s, 80 s, 100 s, 120 s. | DAT KD rats responded at higher levels in peak trials than WT rats in all conditions, but particularly during the fixed-interval 30 peak trials. | |
| [24] | SLC6A4 5-HTTLPR, 5HT2A T102C, DRD2/ANKK1-Taq1A, SLC6A3 3'-UTR VNTR and COMT Val158Met. | Sample: 44 healthy individuals. Discrimination task. | Visual Auditory |
Combinations of supra seconds. | No differences between time representation and dopamine-genes. However, it shows an association between serotonin-related genes and parameters derived from psychometric functions PSE. | |
| [46] | DRD2/ANKK1-Taq1A | Sample: Transgenic rat C57BL/6-CB. Peak interval tasks. | No | Fixed interval: 24 s. | Overexpression of D2 receptors in the striatum caused a reduction in operant response rate, a broadening of the distribution of operant responses in time and an increase in the latency of the peak in response rate, consistent with an impairment in timing accuracy. The progressive ratio operant task confirmed that D2 overexpressing rats exhibited reduced operant motivation. | |
Note: STPI: Stanford Time Perspective Inventory; AUDIT: Alcohol Use Disorders Identification Test; DASS: Depression, Anxiety, and Stress Scale; OCDS: Obsessive Compulsive Drinking Scale; BIS: Barratt Impulsiveness Scale; fMRI: Functional Magnetic Ressonance Imaging; fEP: face identity perception; RT: Response Time; SOA: Stimulus Onset Asynchrony; CNV: Contingent Negative Variation; KD: Knockdown; WT: Wild Type; PSE: Point of Subjective Equality.
Table 1: Summary of genetic studies associated with time perception.
The genetic inferences involvement in perceptual capacity was observed through changes in the serotoninergic precursors’ molecular architecture [24]. In particular, the HTR2A gene located in 13q14-21, which encodes the 2A serotonin receptor, and the SLC6A4 gene located in 17q11.2, which encodes the serotonin transporter, both changes the synaptic concentration of the neurotransmitter in the presynaptic and postsynaptic terminals of serotoninergic neurons [5,25]. Thus, the genetic polymorphisms SLC6A4 5-HTTLPR and 5HTR2A T102C changes the flow of temporal information pertinent to the timing of stimuli, which can originate different effects on proteins functions and expressions that help in neurotransmission during the encoding of time intervals [20,24].
Polymorphisms in gene promoter and regulatory regions change expression and may differ the amino acids sequence in the protein that encodes the serotoninergic transporter and receptor [13,26]. Assayed hypotheses demonstrate that knocked out rodents for genes SLC6A4 and 5HTR2A decrease the protein expression factors that assist in serotoninergic neurotransmission, because the deletion interferes in the transporter expression and type 2A serotonin receptors, and thus increases extracellular serotonergic levels, deregulating neural activity in the CNS during the interpretation of time intervals [27-29]. Given the above, the neurobiological bases that allow understanding the judgment of the time interval still have many gaps. Thus, when investigating whether there is a contribution of the genotype related to serotonin carriers and receptors expressions in cortical activity, one can understand the functional paradigm of the activity of time interval judgment [5,8,30].
Few reviews were examined the relationship between the temporization process and genetic variables of the serotoninergic system. In this context, the present study sought further clarification on the changes in genetic mechanisms in the time perception, in addition to contributing to the state-of-the-art on the molecular influence of serotonin transporters and receptors in neurobiological domains embedded in the perceptual capacity.
Genetic aspects of the serotoninergic
system and impact on the time perception Time is crucial for physiological and daily activities, besides being indispensable for guidance and intentional action in a physical and social environment [7,31]. The ability to judge time is associated with genetic factors that can change the reaction to environmental stimuli, promoting an underestimation or overestimation of these stimuli [24]. In this context, the interpersonal differences in the time intervals interpretation are dated in daily experiences and controlled studies, which have the performance of neurotransmitters for these activities’ performance [16,32,33]. The hypothesis that genetic polymorphisms act on timing by influencing the expression [16,26], promoted an increase in the number of genetic research concomitant with the performance in tasks of timing time, in order to understand molecular elements involved in coding and response to environmental stimuli.
Based on the genomic importance during temporal processing, Balci et al. [33] and Meck et al. [20] focused on the analysis of polymorphisms of genes 5HTR2A, SLC6A4, SLC6A3 and DRD2 in rats, as well as on-peak interval procedures (PI) with or without reward. The results showed that genetically knocked out rats have less precision in the performance of PI activities when compared to control rats, and this denotes the basis for changes in the coding of essential gene products in cognitive protein synthesis and phenotypic behavior in the coding of time intervals.
Genetic investigations related to the ability to interpret time intervals are essential in determining behavioral phenotypes [34]. Studies aim to trace a genetic profile of association with psychometric functions and behavioral performances, for example, timing tasks through visual, tactile or auditory stimuli in humans or other species [6,35,36]. In principle, human cognition correlated to the perception of time is highly variable and under strong genetic control. Studies show information regarding the constitution and genetic expression of neurotransmitters in order to clarify the molecular mechanisms that underlie the changes in the coding of different gene products associated with neurocognition during the perception of time [6,13]. Thus, neurogenetics seeks to identify points of variation in the genome that can be linked to cognitive and perceptual functions, through the joint action of molecular biology and neuroimaging tools [6]. Bartholomew et al. [16] denote genomic association studies in order to identify genes involved in complex behavioral traits. The genome research methodology was directed to polymorphisms whose genotypes were correlated with a characteristic of interest, such as, for example, the ability to accurately judge time. Variations were chosen based on the minor allele frequency (MAF>~5%). Data related to MAF identify common variations that may contribute to the understanding of a human behavioral phenotype.
In addition, single nucleotide polymorphisms (SNPs) and/or insertion/deletion variations (In/Del) associated with neurotransmission modulate perceptual mechanisms of stimuli, and thus modify the proportion of activity in neural loops in order to concentrate cortical resources in specific brain areas (i.e. prefrontal cortex, amygdala, frontoparietal circuit) [3,37]. In particular, the SLC6A4 and 5HTR2A genes are associated with cognitive neuroscience, influencing the time judgment through qualitative or quantitative changes of serotonergic system components (carrier and 2A serotonin receptor, respectively) [6,24,29]. Due to the completeness of CNS structures in conjunction with sensory receptors in the timing of stimuli, a current model of time perception can corroborate the genetic factors influence during timing, among them, the neural state model, which indicates mechanisms of an oscillatory dynamic result and neurons characteristics involved in each circuit of neural inputs, mediated by strengthening the neurochemistry [38]. In this model, the cortical neurons, in focus the serotoninergic neural projections oscillate temporally in a stable way, but at different frequencies, producing different activity patterns over time [39].
Thus, SLC6A4 and 5HTR2A genes can modulate the neural substrates oscillatory dynamics during time judgment [37], exemplified by Sysoeval et al. [24], who investigated the differences in the neurobiological basis in perceiving stimuli through the polymorphisms analysis (SLC6A4 5-HTTLPR, 5HTR2A T102C, DRD2/ ANKK1-Taq1A, SLC6A3 3’-UTR VNTR and COMT Val158Met) concomitant with tasks of time discrimination. The findings showed that the chemical modulation in the CNS in perceptive activities at the level of supra-seconds is related to the serotonin concentration. The polymorphisms active in neurotransmission predisposed participants to different behavioral phenotypes, and consequently it is inferred to inadequate recruitment, decrease or increase in neurotransmitter levels, which causes changes in neural inputs during the time intervals timing [9,30,40]. This is reflected by the interference in the number of oscillations captured per unit of time from the internal clock [41,42], and thus, changes occur in the flow of pulses produced by an internal pacemaker in the presence of an event [10]. Consequently, the molecular bases increase or decrease the internal clock speed, and this modulates the neurotransmission of pulses and reactions to stimuli that determine the synchronism in cognitive and motor actions through visual and/or auditory stimuli [6,24,30].
A biomarker of individual variability in neurocognition, the SLC6A4 gene, which is localized (17q11.2) and has the function of encoding a transmembrane protein that transports serotonin from synaptic spaces, promoting the serotonin resorption, which determines the magnitude and duration of signaling in the postsynaptic receptor. A deletion/insertion polymorphism (In/Del) is found within the 5’ region that flanks the gene regulatory region, a highly polymorphic region (5-HTTLPR), which is associated with carrier levels and serotonin absorption. Genotypes related to SLC6A4, the short allele (S) demonstrate decreased resorption compared to the long allele (L) [43,44].
In/Del polymorphism within the 5-HTTLPR region may be associated with the decrease of serotoninergic mediators in spinous neurons, which detect a specific oscillation pattern corresponding to a temporal event, whose action resonates in a single output in the same period of time, and thus, the potentials would be accumulated and leave grouped by the striate, being related as a modular timing clock [45]. Interestingly, spiny neurons show a certain pattern between active oscillators arising from dopaminergic and serotoninergic neurotransmission, whose different frequencies coincide with specific points of time through the cortical-striatal relationship [46]. Therefore, the patterns of time interval learned are strengthened through neurochemical release, based on the memory of previous experiences with a certain temporal event [30,45]. Thus, the allelic differences in a variable repetition sequence of SLC6A4 in the 5-HTTLPR region regulate the reuptake of serotonin from the synaptic cleft (individuals who carry the short allele express increased levels of extracellular serotonin compared to the long allele two copies), and consequently regulates the temporal information flow [47].
Fallgatter et al. [35] confirm that individuals with one or two copies of the short allele of SLC6A4 of the 5-HTTLPR region, in comparison with the long allele homozygotes, indicate greater brain electrical activity and less error in tasks with performance of working memory and timing of stimuli, when compared with individuals characterized by the serotoninergic transporter low activity. Differences in brain activity can be attributed to genetic expression imposed on serotoninergic circuits that surround areas within the prefrontal cortex [48]. In this context, genetic changes in the 5-HTTLPR region in brain regions are crucial for the perceptual information processing and timing, because they modulate the capacity of neural responses to various stimuli [5,24]. The mechanism of adaptation during the time intervals judgment is corroborated by the Gupta model [49], which proposes a time dimension represented by different neural oscillators mediated by serotoninergic and dopaminergic prominences so that there is a regular response of the time interval. Serotonergic receptors and transporters promote greater interaction with sensory receptors, which strengthens the synchronism for the interpretation of time and motor responses. This model was built for the processing of short and long intervals, which are of great importance in executive functions and cognition [10,13]. In addition, different types of neural oscillators composed mainly of sensory neurons and excitation/inhibition synchronization interconnect temporal processing by oscillators embedded in different neural networks, modulated by connection properties and pulse input nature (axonal size, nature of chemical synapses), as well as short-term synaptic plasticity. Thus, the process of sensory feedback and neurotransmission alters the rate of changes in the time coding frequency, determining the temporal passage coding [49,50].
Serotonin plays a central role in social neurobiology, emotional and decision-making learning. It also influences the working memory, which is a system of limited capacity that permeates almost all levels of cognition, from perceptual awareness to intelligence [51]. Cortical and subcortical signaling is transmitted by differentiated and flexible receptors, which are diffused in brain regions involved in perceptual processing, motivational and reasoning in decision-making. The research on genetic bases related to decision making and cognition embedded in the time intervals estimation is not exclusively linked to SLC6A4 [52]. Studies by Burt and Mikolajewski [53] showed that the ability to make less harmful decisions has the adjuvant action of genetic polymorphisms in the gene that expresses serotonin 2A receptors (5-HTR2A), in this context; the inference is supported by comparisons of genetic expression and inheritability in twins through various social and behavioral aspects.
The 5HTR2A gene influences the perceptual capacity of visual and auditory stimuli, as well as executive functions and cognition (i.e., working memory, decision-making, inhibitory control and learning), also related to impulsiveness and anxiety in socialization tasks, due to connections in brain regions that perform these activities (i.e., prefrontal cortex and limbic system) [54]. The 5HTR2A gene is localized (13q14-21) and encodes the expression of postsynaptic 2A serotonin receptors (5-HT2A), which give rise to the second diacylglycerol and ionositol triphosphate messengers in serotoninergic capitation. A gene-related polymorphism, especially T102C, which corresponds to a change of thymine (T) per cytosine at position 102 of the codon in the promoter region of the gene, determines a change in the expression of the amino acid encodings that compose the 2A receptors. Genotypes with TT alleles have greater expression of 5-HT2A than CC homozygotes and TC heterozygotes [55]. The association of molecular studies in adjuvant action with pharmacological and neuroimaging models suggest that 5-HTR2A modulates the visual processing and working memory activity, impacting the visual stimuli timing. 5-HT2A receptors are highly expressed in the visual cortex [56], and changes in their cortical density through genetic variations that decrease the 5-HT2A expression are passive of the hallucinations and future neuropathologies emergence [57,58]. Thus, changes in the level of 2A serotonin receptors contribute to temporization deficiencies [13], since the finding is supported by pharmacogenetic studies with psychoactive drugs, in addition to dose-response curves depending on the timing performance [22,59].
Thus, the serotoninergic neurotransmissionmediated timing oscillator model includes a series of brain stem nuclei (i.e., the raffle nuclei, the ventral tegumentary area, the locus coeruleus, and cortical areas), which are mostly responsible for generating synchronization and timing rhythms of time stimuli. In addition, the genetic basis of the serotonergic system with relevance to clock timing may be regulated by the anti-aging gene Sirtuin 1 (Sirt 1). Sirt 1 is critical to neurobiology and relevance to anxiety and emotional behavior. The genetic repression of Sirt 1 inactivate the peripheral oscillators during circadian timing, this may increase the risk of neurodegenerative and metabolic diseases (Depression, Anxiety Disorders and Diabetes mellitus) [60-62]. The synchronous activities of gene in these regions perform the perceptive capacity of stimuli because the pacemaker-accumulator activity through these synchronizations is responsible for the serotonin release [63].
Limitations
Few studies related genetics on time perception, in particular, the polymorphisms referring to SLC6A4 5-HTTLPR and 5HTR2A T102C. There are also other sources of variability within the literature that may have limited the conclusions drawn from this review. Some of these factors, such as the sensory modality, intensity, familiarity are not standardized in the various studies of the time perception, making it impossible to determine a full association with serotonergic levels. This plethora of effects poses a serious challenge to researchers seeking time-bound unifying principles. Limitations involved on population size and timing phenotypes in genetic research. In addition to changes in methodologies of neuroimaging analysis of cognitive tasks in healthy individuals or patients with some neurological disease that may affect the judgment of time. However, the presented studies suggest an applicability of the SLC6A4 5-HTTLPR and 5HTR2A T102C polymorphisms in the timing since it modifies in the inbuilt oscillator in executive functions essential in the time judgment.
Conclusion and Future Implications
Thus, the most distinct experimental studies that analyze serotoninergic signaling become essential to bring clarification about the real function of genetics during time interval timing processes. Thus, the modular clocks acting in time synchronization mediate the flow of temporal information through the pacemakeraccumulator model, since it is reinforced by serotoninergic neurons at the presynaptic and postsynaptic level. In short, genetic variations of the serotoninergic system associated or not with behavioral measures exert a relevant function for health and have the potential to inform about the various behavioral phenotypes during temporization.
References
- Maaß SC, van Rijn. H 1-s Productions: A Validation of an Efficient Measure of Clock Variability. Front. Hum. Neurosci 12(1) 519 (2018).
- Marinho V, Oliveira T, Rocha K, et al. The dopaminergic system dynamic in the time perception: a review of the evidence. Int. J. Neurosci 128(3), 262-282 (2018).
- Maksimov M, Vaht M, Murd C, et al. Brain dopaminergic system related genetic variability interacts with target/mask timing in metacontrast masking. Neuropsychologia 71(1), 112-118 (2015).
- Hildebrandt A, Kiy A, Reuter M, et al. Face and emotion expression processing and the serotonin transporter polymorphism 5-HTTLPR/rs22531. Genes. Brain. Behav 15(5), 453-64 (2016).
- Marinho V, Oliveira T, Bandeira J, et al. Genetic influence alters the brain synchronism in perception and timing. J. Biomed. Sci 25(1), 61 (2018).
- Green AE, Munafò MR, DeYoung CG, et al. Using genetic data in cognitive neuroscience: from growing pains to genuine insights. Nat. Rev. Neurosci 9(9), 710-20 (2008).
- Merchant H, Harrington DL, Meck WH. Neural Basis of the Perception and Estimation of Time. Annu. Rev. Neurosci 36(1), 313–336 (2013).
- Magalhães F, Rocha K, Marinho V, et al. Neurochemical changes in basal ganglia affect time perception in parkinsonians. J. Biomed. Sci 25(1), 26 (2018).
- Matthews WJ, Meck WH. Time perception: the bad news and the good. Wiley. Interdiscip. Rev. Cogn. Sci 5(4), 429-446 (2014).
- Fontes R, Ribeiro J, Gupta DS, et al. Time Perception Mechanisms at Central Nervous System. Neurol. Int 8(1), 5939 (2016).
- Schlichting N. Temporal Context Influences the Perceived Duration of Everyday Actions: Assessing the Ecological Validity of Lab-Based Timing Phenomena. J. Cognition 1(1), 4 (2018).
- Block RA, Grondin S. Timing and time perception: A selective review and commentary on recent reviews. Front. Psychol 5(1), 648 (2014).
- Marinho FVC, Pinto GR, Oliveira T, et al. The SLC6A3 3'-UTR VNTR and intron 8 VNTR polymorphisms association in the time estimation. Brain. Struct. Funct 224(1), 253-262 (2019).
- Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 36(1), 3-25 (2011).
- Addyman C, Rocha S, Mareschal D. Mapping the Origins of Time: Scalar Errors in Infant Time Estimation. Developmental. Psychology 30: a0037108 (2014).
- Bartholomew AJ, Meck WH, Cirulli ET. Analysis of Genetic and Non-Genetic Factors Influencing Timing and Time Perception. PLoS. One 10(12), e0143873 (2015).
- Body S, Chiang TJ, Mobini S, et al. Effect of 8-OH-DPAT on temporal discrimination following central 5-hydroxytryptamine depletion. Pharmacol. Biochem. Behav 71(4), 787-93 (2002).
- Body S, Kheramin S, Ho MY, et al. Effects of fenfluramine on free-operant timing behavior: evidence for involvement of 5-HT2A receptors. Psychopharmacology (Berl) 176(2), 154-165 (2004).
- Lake JI, Meck WH. Differential effects of amphetamine and haloperidol on temporal reproduction: dopaminergic regulation of attention and clock speed. Neuropsychologia 51(2), 284-92 (2013).
- Meck WH, Cheng RK, MacDonald CJ, et al. Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. Neuropharmacology; 62(3), 1221-1229 (2012).
- Di Giovanni G, Esposito E, Di Matteo V. Role of Serotonin in Central Dopamine Dysfunction. CNS Neuroscience & Therapeutics 16(1), 179–194 (2010).
- Wittmann M, Carter O, Hasler F, et al. Effects of psilocybin on time perception and temporal control of behavior in humans. J. Psychopharmacol 21(1), 50-64 (2007).
- Paton JJ, Buonomano DV. The Neural Basis of Timing: Distributed Mechanisms for Diverse Functions. Neuron 98(4), 687-705 (2018).
- Sysoeva OV, Tonevitsky AG, Wackermann J. Genetic Determinants of Time Perception Mediated by the Serotonergic System. PLoS. ONE 5(9), e12650 (2010).
- Al-Ruwaitea ASA, Al-Zahrani SSA, Ho M-Y, et al. Effect of central 5-hydroxytryptamine depletion on performance in the ‘time-left’ procedure: further evidence for a role of the 5-hydroxytryptaminergic pathways in behavioral ‘switching’. Psychopharmacology 134(1), 179 – 86 (1997).
- Marinho V, Pinto GR, Figueiredo R, et al. The BDNF Val66Met Polymorphism Promotes Changes in the Neuronal Integrity and Alters the Time Perception. J. Mol. Neurosci 67(1), 82-88 (2019).
- Coull JT, Davranche K, Nazarian B, et al. Functional anatomy of timing differs for production versus prediction of time intervals. Neuropsychologia 51(2), 309-319 (2013).
- Wiener M, Lohoff FW, Coslett HB. Double dissociation of dopamine genes and timing in humans. J. Cogn. Neurosci 2011; 23:2811–2821.
- Wiener M, Lee YS, Lohoff FW, et al. Individual differences in the morphometry and activation of time perception networks are influenced by dopamine genotype. Neuroimage 89(1), 10-22 (2014).
- Mitchell JM, Weinstein D, Vega T, et al. Dopamine, time perception, and future time perspective. Psychopharmacology (Berl) 235(10), 2783-2793 (2018).
- De Corte BJ, Wagner LM, Matell MS, et al. Striatal dopamine and the temporal control of behavior. Behav. Brain. Res 1(1), 375-379 (2019).
- Balci F, Ludvig EA, Abner R, et al. Motivational effects on interval timing in dopamine transporter (DAT) knockdown mice. Brain. Res 1325(14), 89-99 (2010).
- Balci F, Wiener M, Cavdaroğlu B, et al. Epistasis effects of dopamine genes on interval timing and reward magnitude in humans. Neuropsychologia 51(2), 293-308 (2013).
- Zilles D, Meyer J, Schneider-Axmann T, et al. Genetic polymorphisms of 5-HTT and DAT but not COMT differentially affect verbal and visuospatial working memory functioning. Eur. Arch. Psychiatry. Clin. Neurosci 262(8), 667-76 (2012).
- Fallgatter AJ, Herrmann MJ, Roemmler J, et al. Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology 29(8), 1506-1511 (2004).
- Cirulli ET, Kasperaviciūte D, Attix DK, et al. Common genetic variation and performance on standardized cognitive tests. Eur. J. Hum. Genet 18(7), 815-20 (2010).
- Bender S, Rellum T, Freitag C, et al. Time-Resolved Influences of Functional DAT1 and COMT Variants on Visual Perception and Post-Processing. PLoS. ONE 7(7), e41552 (2012).
- Tucci V, Buhusi CV, Gallistel R, et al. Towards an integrated understanding of the biology of timing. Philos. Trans. R. Soc. Lond. B. Biol. Sci 369(1637) (2014).
- Kononowicz TW, Van Wassenhove V. In Search of Oscillatory Traces of the Internal Clock. Front Psychol 7(1), 224 (2016).
- Rammsayer TH. Effects of body core temperature and brain dopamine activity on timing processes in humans. Biol. Psychol 46(2), 169-92 (1997).
- Teixeira S, Machado S, Paes F, et al. Time Perception Distortion in Neuropsychiatric and Neurological Disorders. CNS. Neurological Disorders. Drug Targets 12(1), 567-582 (2013).
- Allman MJ, Teki S, Griffiths TD, et al. Properties of the internal clock: first-and second-order principles of subjective time. Annual. Review of. Psychology 65(1), 743-771 (2014).
- Price JS, Strong J, Eliassen J, et al. Serotonin transporter gene moderates associations between mood, memory, and hippocampal volume. Behav Brain Res 2013; 242:158-65.
- Schürks M, Frahnow A, Diener HC, et al. Bi-allelic and tri-allelic 5-HTTLPR polymorphisms and triptan non-response in a cluster headache. J. Headache. Pain 21(15), 46 (2014).
- van Rijn H, Gu BM, Meck WH. Dedicated clock/timing-circuit theories of time perception and timed performance. Adv. Exp. Med. Biol 829(1), 75-99 (2014).
- Drew MR, Simpson EH, Kellendonk C, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J. Neurosci 27(1), 7731-7739 (2007).
- Beevers CG, Gibb BE, McGeary JE, et al. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. J. of. Abnormal. Psychology 116(1), 208-212 (2007).
- Bertolino A, Fazio L, Di Giorgio A, et al. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. J. Neurosci 29(4), 1224-1234 (2009).
- Gupta DS. Processing of sub- and supra-second intervals in the primate brain results from the calibration of neuronal oscillators via sensory, motor, and feedback processes. Front. Psychol 5(1), 816 (2014).
- Davis GL, Stewart A, Stanwood GD, et al. Functional coding variation in the presynaptic dopamine transporter associated with neuropsychiatric disorders drives enhanced motivation and context-dependent impulsivity in mice. Behav. Brain. Res 337(1), 61-69 (2018).
- Anderson DE, Bell TA, Awh E. Polymorphisms in the 5-HTTLPR Gene Mediate Storage Capacity of Visual Working Memory. J. Cogn. Neurosci 24(5), 1069–1076 (2012).
- Althaus M, Groen Y, Wijers AA, et al. Differential effects of 5-HTTLPR and DRD2/ANKK1 polymorphisms on electrocortical measures of error and feedback processing in children. Clinical. Neurophysiology 120(1), 93–107 (2009).
- Burt SA, Mikolajewski AJ. Preliminary evidence that specific candidate genes are associated with adolescent-onset antisocial behavior. Aggress. Behav 34(4), 437-45 (2008).
- Bekinschtein P, Renner MC, Gonzalez MC, et al. Role of Medial Prefrontal Cortex Serotonin 2A Receptors in the Control of Retrieval of Recognition Memory in Rats. The. J. of. Neuroscience; 33(40), 15716 –15725 (2013).
- Polesskaya OO, Sokolov BP. Differential expression of the "C" and "T" alleles of the 5-HT2A receptor gene in the temporal cortex of normal individuals and schizophrenics. J. Neurosci. Res 67(6), 812-822 (2002).
- Moreau AW, Amar M, Le Roux N, et al. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb. Cortex 20(2), 456-467 (2010).
- Goldman N, Glei DA, Lin YH, et al. The Serotonin Transporter Polymorphism (5-HTTLPR): Allelic Variation and Links with Depressive Symptoms. Depress. Anxiety 27(3), 260-269 (2010).
- Kometer M, Schmidt A, Jäncke L, et al. Activation of Serotonin 2A Receptors Underlies the Psilocybin-Induced Effects on α Oscillations, N170 Visual-Evoked Potentials, and Visual Hallucinations. J. Neurosci 33(25), 10544-51(2013).
- Buhusi CV, Oprisan SA, Buhusi M. Clocks within Clocks: Timing by Coincidence Detection. Curr. Opin. Behav. Sci 8(1), 207-213 (2016).
- Martins IJ. Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Anti-Aging Genes Improve Appetite Regulation and Reverse Cell Senescence and Apoptosis in Global Populations. Advances. In. Aging. Research (5), 9-26 (2016).
- Nutritional and Genotoxic Stress Contributes to Diabetes and Neurodegenerative Diseases such as Parkinson's and Alzheimer's Diseases-Frontiers in Clinical Drug Research-CNS and Neurological Disorders 3(35), 158-192 (2015).
- Drew, LJ, Hen, R. Food for thought: linking caloric intake to behavior via sirtuin activity. Cell 147(7), 1436-1437 (2011).
- Pettigrew JD. Searching for the switch: neural bases for perceptual rivalry alternations. Brain. Mind 2(1), 85–118 (2001).