Research Article - (2018) Volume 8, Issue 3
Yolkin- A Polypeptide Complex Isolated From Chicken Egg Yolk with Potential Neuroprotective and Antioxidative Activity
- Corresponding Author:
- Agnieszka Zabłocka
Laboratory of Signaling Proteins, Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Rudolfa Weigla 12, 53-114 Wrocław, Poland
Tel: +48 71 337 11 72
Fax: +48 71 370 99 30
Abstract
ABSTRACT
Egg yolk proteins are a rich source of nutrients for developing embryos. Among them is vitellogenin, formerly regarded as a female-specific protein, and recently also found in males.
Keywords
Egg Yolk, Yolkin, PC12, Human Whole Blood Cells, ROS, BDNF, Neuroprotection
Introduction
This may indicate that Vg plays a more general function, regardless of gender, making it a particularly interesting protein [1]. The main egg yolk plasma proteins are accompanied by protein fragments released from the C-terminal region of Vg, such as yolk plasma glycoprotein YPG40 and a 42 kDa proteolytic fragment of the vitellogenin I [1-3]. Vitellogenin (Vg) is a phosphoprotein of molecular weight from 250 kDa to 600 kDa present in serum. Vg shows structural similarity in vertebrates and invertebrates. During formulation of the egg, Vg is transported from the plasma into oocytes and then, by receptor endocytosis, proteolytically cleaved into several fragments. Therefore, Vg was considered most of all a source of nutrients for the developing embryos. Vg was initially recognized as a female-specific protein, but recently its presence has also been demonstrated in males. This indicates that Vg in much smaller quantities can play an additional yet unknown biological function, making this protein very interesting [1-4]. Chicken Vg consists of three species, designated as vitellogenins I, II and III [2]. It has been proven that Vt II is enzymatically hydrolyzed into main proteins belonging to the granule fraction of egg yolk namely: lipovitelein I (fragment of N-terminal region), phosvitin (phosphoserylrich domain of Vg) and lipovitelein II. While protein fragments corresponding to the N-terminal Vg domain are well-known, there is no precise identification of the vitellogenin C-terminal region [1,2].
Recent studies have shown the presence of another C-terminal Vg fragment in egg yolk plasma, naturally complexed with immunoglobulin Y [1,4]. This complex, named yolkin, consists of several peptides of apparent molecular weight from over 1 kDa to 35 kDa. Yolkin constituents show high sequence homology to the C-terminal domain of vitellogenin II. The main yolkin fractions (MW about 16, 19, 23, 29, 32 and 35 kDa) are glycoproteins corresponding to the amino acid sequence of vitellogenin starting at position Ala 1572. The minor fractions of MW ranging from about 4 kDa to 12 kDa are free of carbohydrates and start at position Met-1732 in the vitellogenin amino acid sequence [1].
Yolkin is rich in amino-acid residues such as: Asp/ Asn and Glu/Gln. Its polypeptides possess the ability to induce human blood cells to produce cytokines such as interleukin 1β, interleukin 6 and interleukin 10. The complex of IgY and yolkin naturally occurring in the yolk has been shown to have a higher cytokine inducing activity than purified IgY, indicating a new biological function of C terminal Vg fragments. Additionally, among the yolkin constituents, peptides with molecular weights of about 20.4 kDa and 23.2 kDa exert the strongest cytokine IL 6 inducing activity [1]. It has also been shown that yolkin modulates nitric oxide release from mouse macrophages, and down-regulates lipid peroxidation [4,5].
Taken together, the immune-regulatory properties of yolkin indicate it may be a promising bioactive compound to promote neuroprotection and inhibit the progression of dementia in the course of neurodegenerative disorders [1,4]. In this regard, we had previously tested the impact of yolkin on cognitive functions in young and old rats as a model of cognitive decline and the process of brain aging. We had found that yolkin mitigated behavioral symptoms of aging and supported cognitive learning and memory in both groups of rats [6]. In the central nervous system, the low concentration of endogenous antioxidants, the high metabolic rate and the high level of polyunsaturated fatty acids makes this system susceptible to oxidative damage [7,8]. Slow progressive neuronal loss is one of the main features of neurodegenerative diseases, observed in the cerebral cortex of patients with Alzheimer’s disease, in the substantia nigra in Parkinson’s disease, and also during aging [9,10]. One of the key factors in neuron cell death is oxidative stress from the overproduction of highly toxic reactive oxygen species (ROS), leading to through damage to its cell membrane, mitochondrial complexes and DNA. Hydrogen peroxide (H2O2) is one of the main ROS, an endogenous source of hydroxyl free radicals and an inducer of cellular oxidative stress [11]. Considering the pathological role of ROS, it has been a focus of attention in the search for effective therapeutic actions that inhibit or ameliorate neuronal oxidative damage.
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family of growth factors, is a protein that plays a relevant role both in promoting neuronal development, survival and repair following injury, and also in the regulation of synaptic connectivity [12]. Brain and peripheral levels of BDNF may be significantly lower in patients with neurodegenerative disorders compared to healthy subjects [13-15]. Data from the AD and PD animal models also evidence that BDNF may have a protective role on both cholinergic and dopaminergic neurons [16,17]. These data indicate that BDNF is essential for both the survival and activity of dopaminergic and cholinergic neurons in particular brain region. Thus, since patients with PD and AD have reduced peripheral and central levels of this neurotrophin, the disturbance in function may be explained by the negative effect of lower BDNF bioavailability [18]. The role of BDNF in cognition is also well defined [19].
Unfortunately, the successful application of neurotrophins for the treatment of human diseases is still an unresolved problem. One possibility is the use of preparations of natural origin which are able to stimulate cells to release active neuroprotective and immunomodulatory substances. It has been shown that activated human T cells, B cells and monocytes are able to secrete BDNF in vitro. BDNF secreted by immune cells has been shown to be bioactive and supporting neuronal survival [13]. Therefore, the aim of the current work was to evaluate the protective effect of a polypeptide complex, yolkin, against ROS accumulation and its influence on BDNF production by neuronal cells and peripheral human whole blood.
Materials and Methods
▪ Reagents and chemicals
High-glucose Dulbecco’s modified Eagle’s medium (DMEM), phosphate-buffered saline (pH 7.4) (PBS) and trypsin solution were produced by the Laboratory of General Chemistry of the Institute of Immunology and Experimental Therapy, PAS (Poland). L glutamine, antibiotics, (penicillin/streptomycin mixture), donor horse serum and fetal bovine serum (FBS) were from Biowest (Nuaille, France). Bacterial lipopolysaccharide (LPS) from E. coli (serotype 055:B5) and leukoagglutinin (PHA-L), stabilized hydrogen peroxide 30%, 2,7-dichlorofluorescein diacetate (DCFH), 1,1-diphenyl-2-picrylhydrazyl (DPPH), ferrozine and Trolox were from Sigma (St. Louis, MO, USA). 2,4,6,tripyridyl-s-triazine (TPTZ) was from Fluka (Bucharest, Romania). Reagents for SDS-PAGE were from Bio-Rad (California, USA). 2.5S NGF (from mouse submaxillary glands) and BDNF Emax ImmunoAssay System was from Promega (Madison, USA). Human BDNF DuoSet was from R&D System (MN, USA). Page Ruler™ Plus Prestained Protein Ladder (10 kDa - 250 kDa) was obtained from Thermo Scientific (Waltham, MA, USA).
▪ Yolkin preparation
Yolkin was isolated from hen egg yolk plasma according to the procedure described by Polanowski et al. [1].
▪ Cell cultures
PC12 (Tet On) rat pheochromocytoma cells which are widely used as a model to study the cellular mechanisms in neuroprotection/ neurodegeneration, was a gift from Prof. Janusz Matuszyk (Institute of Immunology and Experimental Therapy, PAN, Wrocław). The cells were maintained under 5% CO2 / 95% humidified air at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 5% horse serum and 10% fetal bovine serum, antibiotics (penicillin and streptomycin) and 2 mM L glutamine, with the culture medium changed once every three days.
Whole blood samples used as ex vivo experimental model mimics the natural environment of immunocompetent cells and preserves the intercellular communications between the different blood cell populations. Blood samples from healthy donors were kindly provided by the Station of Blood Donation, 4th Military Hospital, Wrocław, Poland. Blood samples were collected into syringes containing 10 U/ml of heparin. Within 2 h of collection, the blood was diluted 10 fold with RPMI 1640 medium supplemented with 100 units/ml penicillin, 100 mg/ml streptomycin and 0.5 mg/ml L glutamine. Whole blood samples were used for the determination of peripheral BDNF.
▪ BDNF induction and determination
PC12 stimulation. PC12 Tet On cells (1x106/ ml) were suspended in serum-free DMEM medium and plated in 6-well culture plates. Yolkin at concentrations of 1 μg/ml, 10 μg/ ml, 100 μg/ml and 150 μg/ml was applied to the cells and incubated for 6 h at 37 °C in a 5% CO2 atmosphere to induce BDNF production. BDNF level was measured in supernatants by ELISA test using the BDNF Emax ImmunoAssay System (Promega).
Human whole blood. The experiments were performed according to the procedure described by Inglot et al. [20]. One ml portions of whole blood diluted 10-fold with RPMI 1640 medium were distributed into 24-well flat-bottomed tissue culture plates. Polypeptide yolkin complex (1 μg/ml - 100 μg/ml) was added to whole blood. As a reference, positive inducers LPS and PHA (2+2 μg/ml) were used. Non-stimulated blood samples were used to measure the spontaneous production of cytokines (negative control). The plates were incubated for 24 h at 37 °C in a 5% CO2 atmosphere. The supernatants were collected and used for BDNF determination by ELISA using Human BDNF DuoSet (R&D System).
▪ Assay of cell viability
Cell viability was evaluated by MTT assay [21]. In brief, the PC12 cells were seeded onto poly- L-lysine-coated 96 well plates (1x104/well) and next incubated for 4 h and 24 h with inducers: yolkin (1 μg/ml - 150 μg/ml) or hydrogen peroxide (10 μM - 300 μM). The MTT (5 mg/ ml) solution was added to each culture well for further incubation. After 4 h/24 h, the culture medium was removed and the formazan crystals were dissolved by the addition of 100 μl DMSO to each well, along with vigorous shaking to complete solubilization. Finally, the absorbance was measured with an Enspire™ 2300 microplate reader (Parkin Elmer, Massachusetts, USA) at 570 nm. Cell viability was expressed as the percentage of living cells incubated with inducers vs control.
▪ Analysis of neurite outgrowth
PC12 cells (1×104/well) were plated onto poly- L-lysine-coated chamber slides (Nunc) and cultured in medium supplemented with 1% horse serum. Yolkin at concentration ranges from 1 μg/ml to 150 μg/ml was added to the cells as potential inducer of neuritogenesis. NGF (0.1 μg/ml) was used as a positive control, while untreated PC12 cells were used as a negative control. PC12 cells were maintained at 37 °C in a humidified atmosphere of 95% air 5% CO2 for 3 10 days. Cells were observed by phasecontrast microscopy and the number of neuritepositive cells counted.
▪ Detection of intracellular ROS accumulation
Intracellular H2O2 and low-molecular weight peroxides are able to oxidize 2’,7’-dichlorofluorescin diacetate (DCFHDA) to dichlorofluorescein (DCF), which is highly fluorescent under absorption analysis [22]. In brief, PC12 cells (104/well) were plated onto 96-well poly-L-Lysine–coated plates 24 h before experiments. Next, the medium was removed, the cells washed with culture medium and then incubated with 50 μM DCFH-DA in DMEM supplemented with 1% FBS (loading solution) for 30 min in 5% CO2 at 37 ˚C. After DCFH DA was removed, the cells were washed twice and incubated with loading solution containing H2O2 as a free radical generator. To determine the regulatory effect on free oxygen radicals generation, yolkin (1 μg/ml - 150 μg/ ml) was applied to the cells one hour before or simultaneously with H2O2. Subsequently, the DCF fluorescence was measured using an Enspire™ 2300 microplate reader (Parkin Elmer, Massachusetts, USA) at excitation and emission wavelengths of 485 nm and 530 nm, respectively.
▪ Determination of antioxidant activity as the ability to scavenge DPPH free radicals
The antioxidant activity of yolkin was assessed on the basis of the radical scavenging effect on stable 1,1-diphenyl-2-picrylhydrazyl free radical according to Yen and Chen [23], with minor modifications. The tested samples were dissolved in water to a final volume of 1 ml and mixed with 1 ml of ethanol (98%). The reaction was started by adding 0.5 ml of 0.3 mM DPPH in ethanol. The mixtures were left for 30 minutes at room temperature and the absorbance of the resulting solutions measured at 517 nm. For calibration, aqueous solutions of known Trolox concentrations ranging from 2 μg to 20 μg (able to scavenge 500 μL of 0.3 mM DPPH radical solution) were used. Radical scavenging activity of the peptides was expressed as µM troloxeq
▪ FRAP method
The FRAP method (Ferric Reducing Antioxidant Power) was used to determine the antioxidative capacity of yolkin according to Benzie and Strain [24]. 3 ml of FRAP working solution (300 mM acetate buffer pH 3.6; 10 mM 2,4,6,tripyridyl-striazine and 20 mM FeCl3 x 6 H2O (10:1:1 v/v)) was mixed with 1 ml of the sample. After 10 min of reaction, the absorbance was measured at λ = 593 nm. An aqueous solution of known Fe (II) concentration was used for calibration. Results were expressed as μg Fe2+.
▪ Determination of Fe (II) ion chelation
Chelation of iron ions by yolkin was estimated by the method of Xu et al. [25] with some modifications. A 250 μl sample was mixed with 1250 μl H2O and 110 μl 1 mM FeCl2. After 2 min, 1 ml of 500 μM ferrozine aqueous solution was added and the mixture was allowed to react for 10 minutes. The absorbance of ferrous iron-ferrozine complex was measured spectrophotometrically at 562 nm. A known concentration of FeCl2 (0 μg 20 μg) was used to generate a standard curve and the ability to chelate iron ions was expressed as μg Fe2+.
▪ Statistical analysis
Statistical analyses were performed using the software package Statistica 6 by StatSoft. Data were expressed as means ± SD or medians with quartiles, minimums and maximums. Statistical significance of differences between the values of analyzed samples was evaluated by nonparametric Wilcoxon test and Student’s t test. A value of *p ≤ 0.05 was considered statistically significant.
Results
▪ Effect of yolkin on neurite extension
PC12 cells were incubated on poly-L-lysinecoated chamber slides (Nunc) with various concentrations of yolkin (1 µg/ml - 150 µg/ml). NGF (0.1 µg/ml) was used as a positive control. After 7 days of cultivation the number of cells with neurites was estimated among 50 cells in a phase-contrast microscope field. PC12 control cells were round without neurites. Neurite outgrowth was observed in at least 80% of NGF treated cells. No neuritogenic effect was observed after yolkin application (data not shown).
▪ Effect of yolkin on PC 12 cells viability
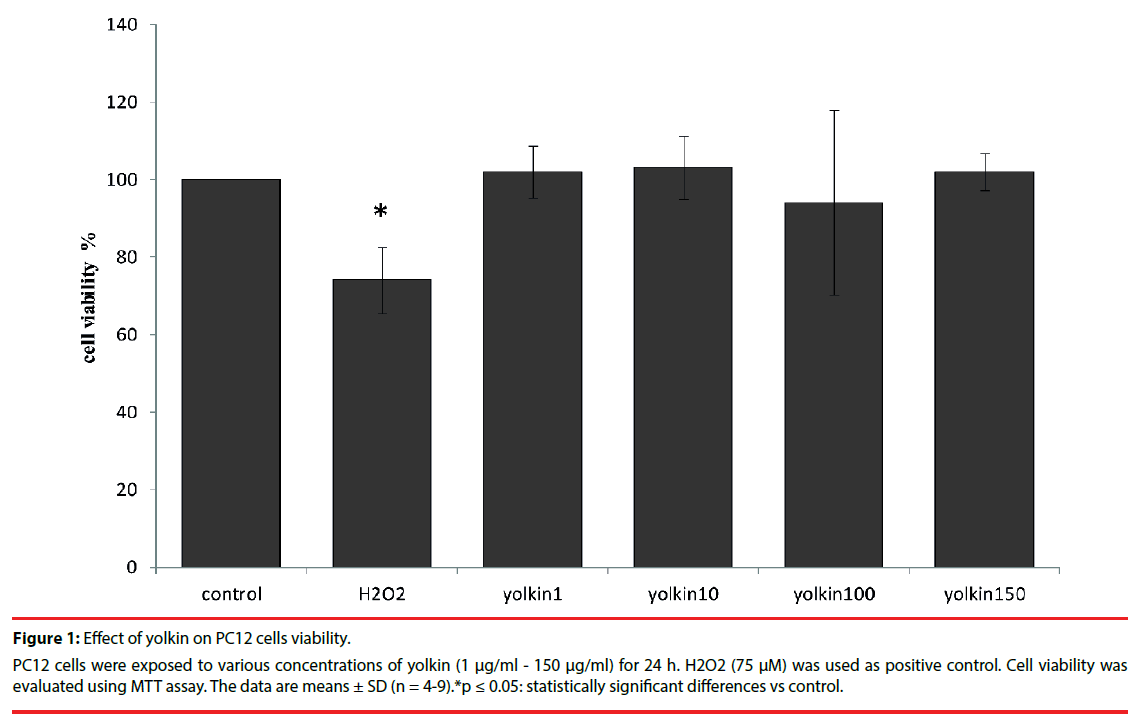
Treatment of PC12 cells with yolkin (1 µg/ml - 150 µg/ml) for 24 h did not show any toxic effect (Figure 1), thus indicating that yolkin was not toxic even at the highest concentration used in this study.
Figure 1: Effect of yolkin on PC12 cells viability.
PC12 cells were exposed to various concentrations of yolkin (1 μg/ml - 150 μg/ml) for 24 h. H2O2 (75 μM) was used as positive control. Cell viability was evaluated using MTT assay. The data are means ± SD (n = 4-9).*p ≤ 0.05: statistically significant differences vs control.
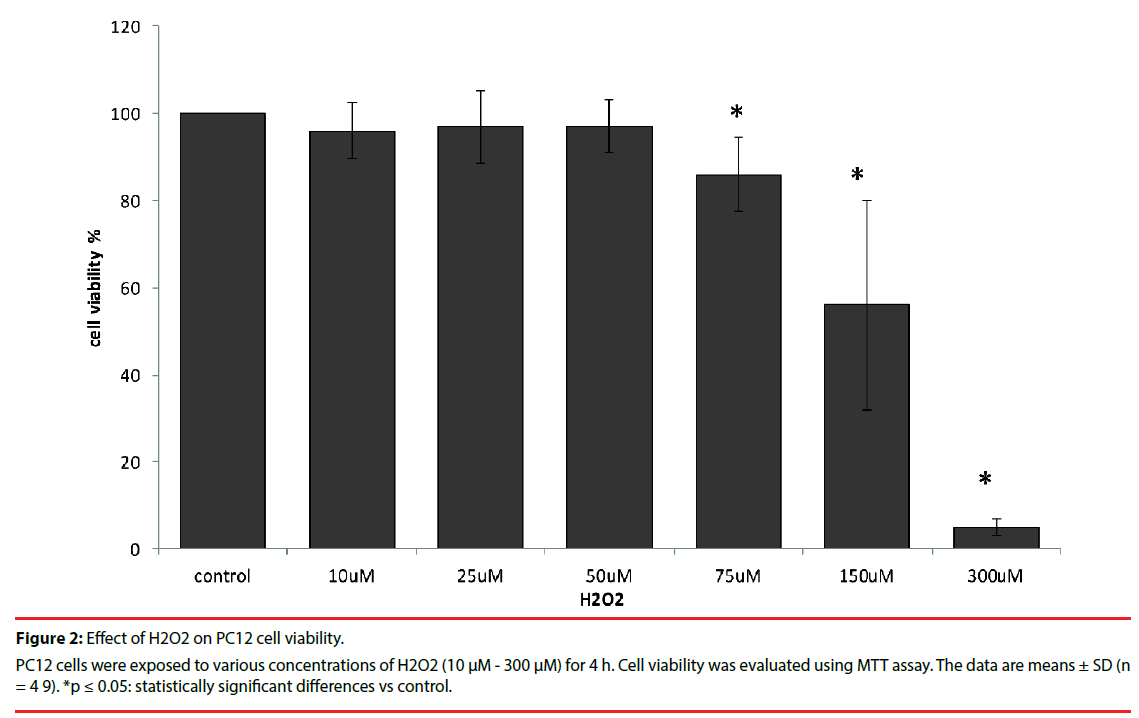
As is shown in Figure 2 H2O2 at doses ranging from 10 µM to 50 µM had no inhibitory effect on PC12 cell viability. However, after 4 h incubation with 75 µM H2O2 viability decreased to 86%. Exposure to 300 µM H2O2 caused significant death (95%) in PC12 cell culture in vitro.
▪ Effect of yolkin on the level of H2O2– induced oxidative stress
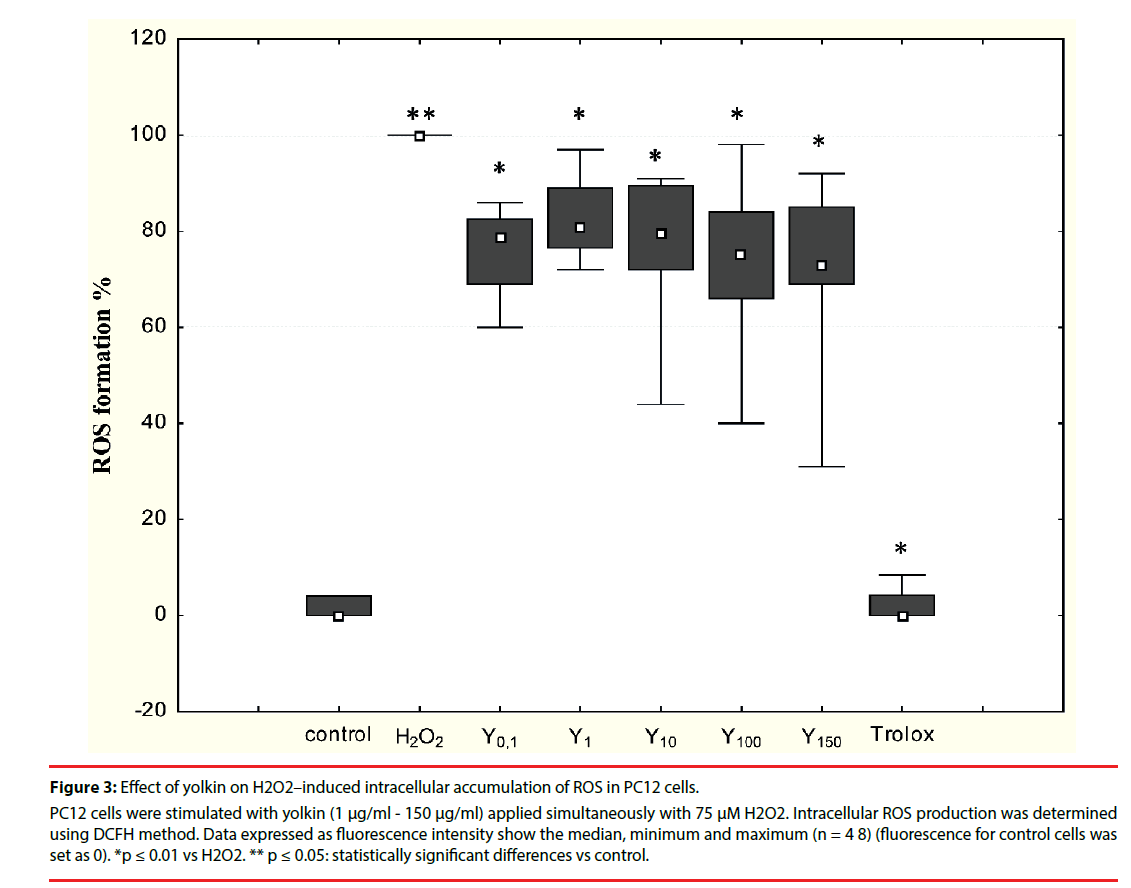
It was shown in preliminary experiments that yolkin (1 μM - 150 μM) applied to the PC12 cells alone is not an inducer of free oxygen radicals (data not shown). A time-dependent increase in free radical production had been observed in cells treated with 75 μM H2O2 compared with control cells (data not shown). To determine the impact of yolkin on oxidative stress inhibition, PC12 cells were simultaneously treated with 75 μM H2O2 and yolkin at doses 1 µg/ml - 150 µg/ ml for 30 min. Yolkin significantly decreased intracellular ROS generation (as a result of H2O2 toxicity) to 81%, 79%, 75% and 73%, respectively (Figure 3). No inhibitory effect was observed when yolkin was applied to the cells one hour before H2O2 application (data not shown).
Figure 3: Effect of yolkin on H2O2–induced intracellular accumulation of ROS in PC12 cells.
PC12 cells were stimulated with yolkin (1 μg/ml - 150 μg/ml) applied simultaneously with 75 μM H2O2. Intracellular ROS production was determined using DCFH method. Data expressed as fluorescence intensity show the median, minimum and maximum (n = 4 8) (fluorescence for control cells was set as 0). *p ≤ 0.01 vs H2O2. ** p ≤ 0.05: statistically significant differences vs control.
▪ Antioxidant properties of yolkin
As it is presented in Table 1, yolkin did not show antioxidant activity. No impact on DPPH scavenging activity nor ferric reducing activity was observed (FRAP). Only a very weak dose-dependent ability to chelate ferrous ions was observed.
| Amount of yolkin [µg] |
DPPH scavenging activity [µM Troloxeq] |
Ferrous ion-chelating activity [µg Fe2+] |
Ferric reducing ability (FRAP) [µg Fe2+] |
|---|---|---|---|
| 0.1 | 0.005± 0.004 | 0± 0 | 0± 0 |
| 1.0 | 0.007± 0.001 | 0.157± 0.136 | 0± 0 |
| 10 | 0.006± 0.001 | 0.157± 0.272 | 0± 0 |
| 100 | 0± 0 | 1.396± 0.165 | 0.015± 0.007 |
| 150 | 0.016± 0.001 | 3.248± 1.712 | 0.040± 0.020 |
Table 1: Antioxidant activity of yolkin isolated from egg yolk plasma (tests in vitro) All data are expressed as mean values (mean ± SD, n = 3).
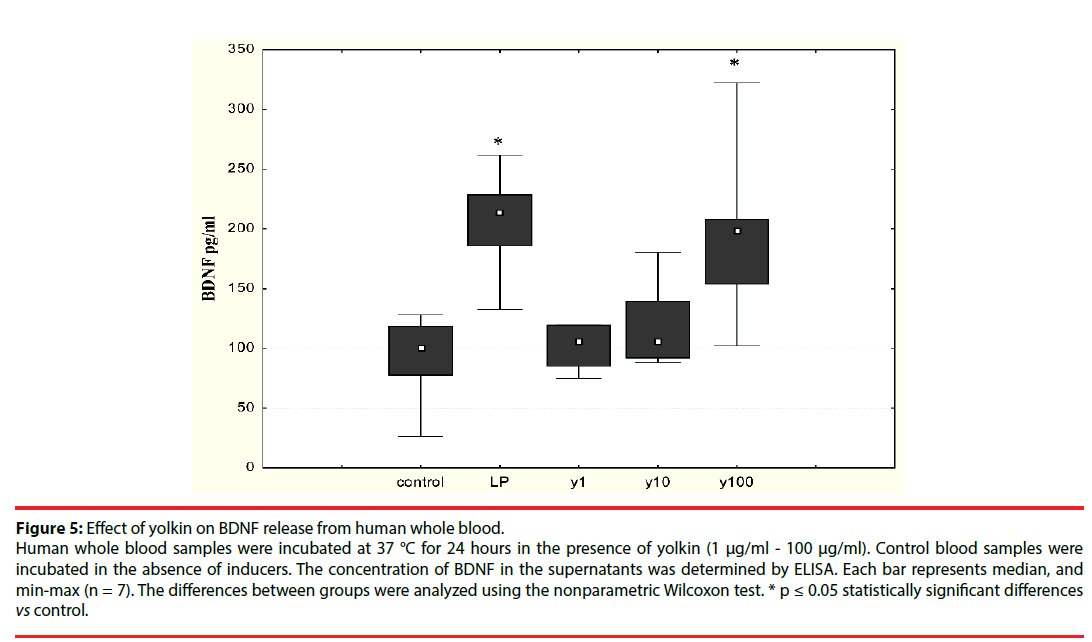
▪ Effect of yolkin on BDNF production
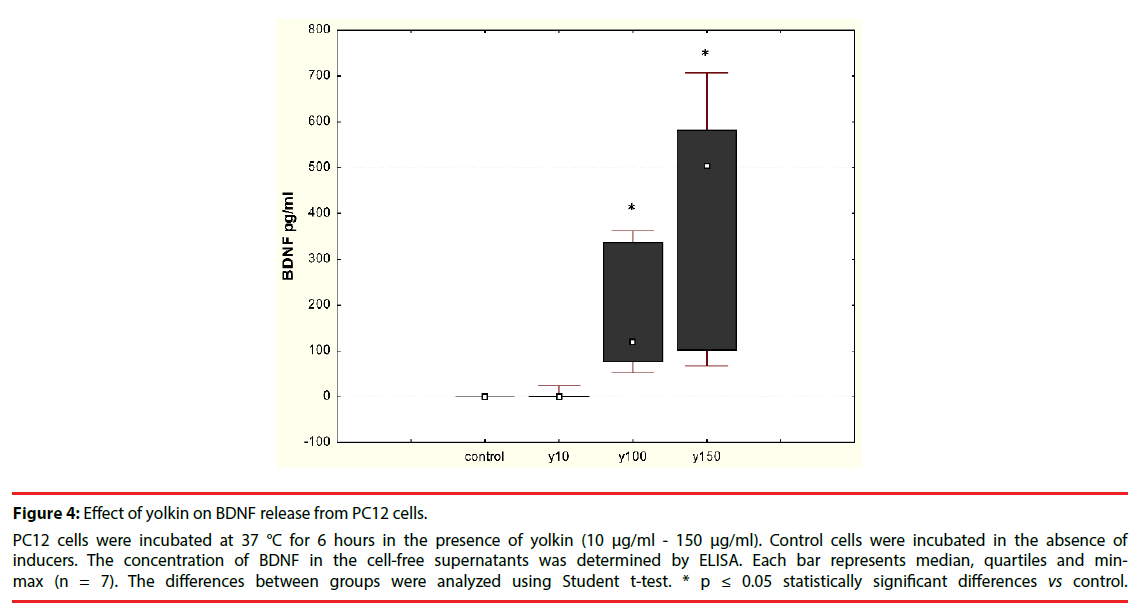
The effect of the polypeptide complex yolkin on mature BDNF release was determined by ELISA assay. It was shown that yolkin at doses 100 μg/ml and 150 μg/ml stimulated the release of significant amounts of BDNF in both neuronal PC12 cells (Figure 4) and human peripheral whole blood (Figure 5).
Figure 4: Effect of yolkin on BDNF release from PC12 cells.
PC12 cells were incubated at 37 °C for 6 hours in the presence of yolkin (10 μg/ml - 150 μg/ml). Control cells were incubated in the absence of inducers. The concentration of BDNF in the cell-free supernatants was determined by ELISA. Each bar represents median, quartiles and minmax (n = 7). The differences between groups were analyzed using Student t-test. * p ≤ 0.05 statistically significant differences vs control.
Figure 5: Effect of yolkin on BDNF release from human whole blood.
Human whole blood samples were incubated at 37 °C for 24 hours in the presence of yolkin (1 μg/ml - 100 μg/ml). Control blood samples were incubated in the absence of inducers. The concentration of BDNF in the supernatants was determined by ELISA. Each bar represents median, and min-max (n = 7). The differences between groups were analyzed using the nonparametric Wilcoxon test. * p ≤ 0.05 statistically significant differences vs control.
Discussion
In 2012, it was demonstrated for the first time by Polanowski et al. [4] that the main chicken egg immunoglobulin IgY is accompanied by an additional polypeptide fraction named yolkin. Yolkin occurs as a complex of vitellogenin II-derived peptides with a molecular weight from 1 kDa to 35 kDa.
The mechanism of action of yolkin is still under investigation. The recently obtained results demonstrate that yolkin possesses neuroprotective activity by counteracting neuronal injury induced by H2O2 in PC12 cells and up-regulating production of BDNF by neuronal PC12 cells and by human whole blood. Increased production of reactive oxygen species (ROS) is a key component of the pathogenesis of neurodegenerative diseases [7,8,26-28]. Excessive production of ROS causes oxidative damage to cellular proteins, lipids and nucleic acids, and finally leads to apoptosis or necrosis in cells. Hydrogen peroxide (H2O2) has been identified in post-mortem brains as an important mediator of neuronal cell death in neurodegenerative diseases. It is a small molecule which is able to diffuse easily through the cell membrane and generate toxic hydroxyl free radicals and cellular oxidative stress [11,27].
Therefore, we studied the neuroprotective activity of polypeptide complex yolkin isolated from chicken egg yolk and its ability to reduce oxidative stress. The experiments were performed on the PC12 cell line which is commonly used as a screening model for testing the prevention of ROS–induced neuronal injury [29,30]. We showed that treatment of PC12 cells with 75 µM H2O2 for 2 hours induced moderate oxidative stress (Figure 2). Yolkin, in a dose-dependent manner, significantly reduced intracellular level of ROS generated in PC12 cell after treatment with 75 µM H2O2 (Fig. 3). To check the antioxidant effect of yolkin, its scavenging activity (DPPH), ferric reducing activity (FRAP) and ferrous ionchelating activity was determined. As shown in Table 1, yolkin did not have antioxidant activity. Therefore, the observed significant reduction of the intracellular ROS in PC12 cells after yolkin application can indicate its effect on regulation of the endogenous antioxidant system activity (including catalase, superoxide dismutase and also glutathione). Similar properties in regulation of the antioxidant system and inhibition of free oxygen radicals synthesis had been previously shown in the case of proline-rich polypeptide complex PRP accompanying IgG fraction in colostrum [31-33].
Brain-derived neurotrophic factor (BDNF) is widely expressed in both a developing and mature brain. It plays a crucial role both in promoting neuron development, survival and repair after injury and also in regulation of synaptic activity and plasticity [12,34-36]. In humans, the levels of this neurotrophin in central and peripheral neurons may be significantly lower in patients with neurodegenerative disorders compared to healthy subjects [13-15]. In this way, in patients with PD and AD with reduced levels of this neurotrophin, the disturbance in neuronal function may be explained by the negative effect of decreased BDNF bioavailability [18].
Neurons are an important cellular source of BDNF. It was also shown that activated human T cells, B cells and monocytes are able to secrete BDNF in vitro. BDNF secreted by immune cells is bioactive and supports neuronal survival in vitro [13]. However, the use of neurotrophins for the treatment of human diseases is still an unresolved problem. One successful application is the use of preparations of natural origin that are able to penetrate the BBB and which are not toxic and are able to stimulate cells to release active neuroprotective and immunomodulatory substances.
Because BDNF is now considered a potential therapeutic agent for human neurodegenerative diseases, we decided to study the ability of yolkin to stimulate both neuronal and human whole blood to produce BDNF. Our present results showed that yolkin stimulated both neuronal (Figure 4) and peripheral whole blood (Figure 5) to release significant amounts of mature BDNF, when added at concentrations higher than 10 µg/ml.
Inflammation accompanying neurodegenerative processes provides increased permeability of the blood-brain barrier for peripheral blood cells like T lymphocytes. Therefore, BDNF produced by immune cells stimulated by yolkin complex can be helpful to minimize neuronal damage in the CNS.
In summary, yolkin may directly activate neuronal PC12 cell to produce and release BDNF and may indirectly support neuronal protection by activation of peripheral immune cells to release BDNF. In young and old rats used as a model of cognitive decline and the process of brain aging, it was observed that yolkin mitigated the behavioral symptoms of aging and supported cognitive learning and memory in rats from both age groups [6]. On the base of these results, we can hypothesize that the neuroprotective effect of yolkin may be associated with its effect on CNS cells and also the cells of peripheral blood which flow to the brain through the damaged blood-brain barrier (BBB).
The impact of yolkin on BDNF production/release and on decrease of intracellular ROS level generated in response to oxidative stress may suggests its neuroprotective abilities and shed some light on the possible mechanism of yolkin action both on the periphery and in the central nervous system. We proposed two possible mechanisms of yolkin action. We speculated that yolkin can be CREBdependent cellular signaling activator providing to BDNF expression/production. Endogenously synthetized BDNF is stored and transported in dense core vesicles and secreted at synapses in response to activity [37,38]. Therefore, effect of yolkin on molecular mechanisms regulating BDNF transport and release from synapses is also considered. We can also speculate that the effect of yolkin on decrease of intracellular ROS level generated in response to oxidative stress may indicate its impact on antioxidant enzymes activity and glutathione level.
Conclusion
In conclusion, this study showed that yolkin isolated from chicken egg yolk had no neuritogenic activity. However, it demonstrated a potent neuroprotective effect against H2O2– induced PC12 cell damage. This effect was connected with attenuation of oxidative stress and stimulating neurotrophin BDNF production by both neurons and human whole blood, thus making BDNF more available to neurons and in turn support the survival and function of neuronal cells. It indicate that yolkin is able to amplify neuroprotective mechanisms in the CNS and can considered as a potent therapeutic agent in the treatment of neurodegenerative diseases.
Acknowledgements
The project is co-financed by the European Regional Development Fund within the Innovative Economy 2007-2013 Operational Programme. project no. POIG.01.03.01-00-133/08 – “Innovative technologies of production of biopreparations based on new generation egg (OVOCURA)” and by the Wroclaw Centre of Biotechnology program “The Leading National Research Centre (KNOW) 2014-2018”.
Conflict of Interest
The authors declare no conflict of interest.
References
- Polanowski A, Sosnowska A, Zabłocka A, et al. Immunologically active peptides that accompany hen egg yolk IgY - separation and identification. Biol. Chem 394(1), 879-887 (2013).
- Yamamura J, Adachi T, Aoki N, et al. Precursor-product relationship between chicken vitellogenin and the yolk proteins: the 40 kDa yolk plasma glycoprotein is derived from the C-terminal cysteine-rich domain of vitellogenin II. Biochim. Biophys. Acta 1244(1), 384-94 (1995).
- Kim HK, Lee S, Leem KH. Protective effect of egg yolk peptide on bone metabolism. Menopause 18(1), 307-313 (2011).
- Polanowski A, Zabłocka A, Sosnowska A, et al. Immunoregulatory activity accompanying chicken egg yolk immunoglobulin Y. Poultry. Sci 91(1), 3091-3096 (2012).
- Zabłocka A, Sosnowska A, Urbaniak A, et al. Peptides accompanying chicken egg yolk IgY – alternative methods of isolation and immunoregulatory activity. Food. Function 5(1), 724-733 (2014).
- Lemieszewska M, Jakubik-Witkowska M, Stańczykiewicz B, et al. Pro-Cognitive Properties of the Immunomodulatory Polypeptide Complex, Yolkin, from Chicken Egg Yolk and Colostrum-Derived Substances: Analyses Based on Animal Model of Age-Related Cognitive Deficits. Arch. Immunol. Ther. Exp 64(1), 425-434 (2016).
- Dasuri K, Zhang I, Keller JN. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free. Radic. Biol. Med 62(1), 170-185 (2013).
- Smith JA, Park S, Krause JS, et al. Oxidative stress, DNA damage and telomeric complex as therapeutic targets in acute neurodegeneration. Neurochem. Int. 62(1), 764-775 (2013).
- Torrăo AS, Café-Mendes CC, Real CC, et al. Different approaches, one target: understanding cellular mechanisms of Parkinson’s and Alzheimer’s diseases. Rev. Bras. Psiquiatr 34(1), S194-S205 (2012).
- Zhao Y, Zhao B. (2013) Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell Longev. 316523 (2013).
- Halliwel B. Free radicals and antioxidants: updating a personal view. Nutr. Rev 70(1), 257-265 (2012).
- Calabrese F, Rossetti AC, Racagni G, et al. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Frontiers. Cell. Neurosci 430(1), 1-7 (2014).
- Kerschensteiner M, Gallmeyer E, Behrens L, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J. Exp. Med 189(1), 865-870 (1999).
- Scalzo P, Kümmer A, Bretas TL, et al. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J. Neurol 257(1), 540-545 (2010).
- Sopova K, Gatsiou K, Stellos K, et al. Dysregulation of neurotrophic and haematopoietic growth factors in Alzheimer’s disease: from pathophysiology to novel treatment strategies. Curr. Alzheimer. Res 11(1), 27-39 (2014).
- Galpern WR, Frim DM, Tatter SB, et al. Cell mediated delivery of brain-derived neurotrophic factor enhances dopamine levels in an MPP+ rat model of substantia nigra degeneration. Cell. Transplant 5(1), 225-232 (1996).
- Shults CW, Kimber T, Altar CA, et al. BDNF attenuates the effect of intrastriatal injection of 6-hydroksydopamine. NeuroReports 6(1), 1109-1112 (1995).
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine COMT and BDNF. Genes. Brain. Behav 5(1), 311-328 (2006).
- Sakata K, Martinowich K, Woo NH, et al. Role of activity-dependent BDNF expression in hippocampal- prefrontal cortical regulation of behavioral perseverance. Proc. Natl. Acad. Sci. USA. 110, 15103-15108 (2013).
- Inglot AD, Janusz M, Lisowski J. Colostrinin: a proline-rich polypeptide complex from ovine colostrum is a modest cytokine inducer in humans leucocytes. Arch. Immunol. Ther. Exp. 44(1), 215-224 (1996).
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 65(1), 55-63 (1983).
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free. Rad. Biol. Med. 27(1), 612-616 (1999).
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagencity. J Agric. Food. Chem 43(1), 27-32 (1995).
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal. Biochem 293(1), 70-76 (1996).
- Xu X, Katayama S, Mine Y. Antioxidant activity of tryptic digests of hen egg yolk phosvitin. J. Sci. Food. Agric 87(1), 2604-260 (2007).
- Tufail M, Takeda M. Molecular characteristics of insect vitellogenins. J. Insect. Physiol. 54(1), 1447-1458 (2008).
- Kim GH, Kim JE, Rhie SJ, et al. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol 24(1), 325-340 (2015).
- Chiurchiu V, Orlacchio A, Maccarrone M. Is modulation of oxidative stress an answer? The state of the art of redox therapeutics in neurodegenerative diseases. Oxid. Med. Cel. Longev, 7909380(1), 1-11 (2016).
- Ao GZ, Chu XJ, Ji YY, et al. Antioxidant properties and PC12 cell protective effects of a novel curcumin analogue (2E,6E)-2-6-Bis(3,5-dimethoxybenzylidene) cyclohexanone (MCH). Int. J. Mol. Sci. 15, 3970-3988 (2014).
- Ogura Y, Sato K, Kawashima K, et al. Subtoxic levels of hydrogen peroxide induce brain-derived neurotrophic factor expression to protect PC12 cells. BMC Research Notes 840(1), 1-8 (2014).
- Zabłocka A, Janusz M, Macała J, et al. A proline-rich polypeptide complex (PRP) isolated from ovine colostrum. Modulation of H2O2 and cytokine induction in human leukocytes. Int. Immunopharmacol 7(1), 981-988 (2007).
- Zabłocka A, Siednienko J, Mitkiewicz M, et al. Proline-rich polypeptide complex (PRP) regulates secretion of inflammatory mediators by its effect on NF-қB activity. Biomed. Pharmacother 64(1), 16-20 (2010).
- Zabłocka A, Janusz M. Effect of proline-rich polypeptide complex Colostrinin™ on the enzymatic antioxidant system. Arch. Immunol. Exp. Ther 60(1), 383-390 (2012).
- Waterhouse EG, Xu B. New insight into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol. Cell. Neurosci 42(1), 81-89 (2009).
- Lu Y, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and disfunction. Handb. Exp. Pharmacol 220(1), 223-250 (2014).
- Hachem LD, Mothe AJ, Tator CH. Effect of BDNF and other potential survival factors in model of in vitro oxidative stress on adult spinal cord-derived neural stem/progenitor cells. BioRes. Open. Access 4.1(1), 146-159 (2015).
- Wong YH, Lee CM, Xie W, et al. Activity-dependent BDNF release via endocytic pathway is regulated by synaptotagmin-6 and complexin. PNAS E4475-E4484 (2015).
- Al-Qudah MA, Al-Dwairi A. Mechanisms and regulation of neurotrophin synthesis and secretion. Neurosci 21(1), 306-313 (2016).