Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Hippocampal Low-Frequency Stimulation Decreased cAMP Response Element-Binding Protein and Increased GABAA Receptor Subunit α1 in Amygdala-Kindled Pharmacoresistant Epileptic Rats
- Corresponding Author:
- Guofeng Wu
Department of Neurology
Affiliated Hospital of Guizhou Medical University, China
Tel: +86-1380- 9431-723
Fax number: +86-0851-6850-654
Abstract
Abstract
Aim: To observe the changes in cAMP response element-binding protein (CREB) and GABAA receptor subunit α1 in amygdala-kindled pharmacoresistant epileptic rats subjected to hippocampal low-frequency stimulation (Hip-LFS).
Methods: A total of sixteen pharmacoresistant epileptic rats were selected from fifty rats with amygdalakindled epilepsy by testing their seizure response to phenytoin (PHT) and phenobarbital (PB). The pharmacoresistant epileptic rats were assigned to a pharmacoresistant control group (PRC group) or a pharmacoresistant stimulation group (PRS group), each of which included 8 rats. The same number of pharmacosensitive epileptic rats was assigned to a pharmacosensitive control group (PSC group, 8 rats). A normal rat control (NRC) group of 8 rats was also used in the study. A stimulation electrode was implanted in the hippocampus of each rat from each of the four groups. Hip-LFS was administered to the PRS group twice per day for 2 weeks. The other three groups only received hippocampal sham stimulation. Following 2 weeks of Hip- LFS, seizure degree and amygdala EEG were recorded. The levels of CREB protein and GABAA receptor subunit α1 in the hippocampal tissues were measured by a Western blot.
Results:The CREB level increased remarkably and the GABAA receptor subunit α1 level decreased in the pharmacoresistant epileptic rats compared with the control. Following two weeks of Hip-LFS, stimulation-induced seizures and amygdala EEG were inhibited significantly. The CREB and p-CREB level decreased significantly in the PRS group compared with the PRC group. However, the GABAA α1 subunit level in the PRS group increased dramatically compared with that in the PRC group.
Conclusions: Hip-LFS might increase the expression of the GABAA α1 subunit and decrease the level of CREB. The Hip-LFS-induced increase in the level of the GABAA receptor α1 subunit might be associated with the decreases in CREB and p-CREB in the treatment of amygdala-kindled pharmacoresistant epileptic rats.
Keywords
Pharmacoresistance, Hippocampal stimulation, Amygdala, cAMP response element-binding protein, GABAPharmacoresistance, Hippocampal stimulation, Amygdala, cAMP response element-binding protein, GABAA receptors receptors
Abbreviations
CREB: cAMP response element-binding protein; p-CREB: Phosphorylated CREB; GABA: Gamma-aminobutyric acid; AEDs: Antiepileptic drugs; TLE: Temporal lobe epilepsy; DBS: Deep brain stimulation; PHT: Phenytoin sodium injection; PB: Phenobarbital; NRC: Normal rat control; PSC: Pharmacosensitive control; PRC: Pharmacoresistant control; PRS: Pharmacoresistant stimulation; ADT: Afterdischarge threshold; EEG: Electroencephalogram; Hip-LFS: Hippocampal low-frequency stimulation; IOD: Integrated optical density
Introduction
Epilepsy is considered a major public health problem that diminishes health-related quality of life [1]. Although many patients with epilepsy take advantage of the large number of available antiepileptic drugs (AEDs) and several new ones are under development, 35%-40% of patients with epilepsy remain resistant to pharmacotherapy [2].
Temporal lobe epilepsy (TLE) is the most common type of pharmacoresistant epilepsy. In many patients with TLE, favourable outcomes might be achieved from traditional surgery by selectively removing the anterior part of the lobe or the amygdala-hippocampus. However, some patients with TLE are unsuitable candidates for epilepsy surgery, either because of the bilateral nature of the disease or because of concerns for major postoperative verbal memory loss after removal of the hippocampus [3]. These patients may be amenable to hippocampal deep brain stimulation (DBS). DBS has emerged as a potential therapeutic strategy in the treatment of neurological disorders including epilepsy [4]. Clinical and experimental studies have demonstrated hippocampal DBS to be effective in the treatment of intractable epilepsy [5-12]. A multicentre study in Europe has recently compared the safety and efficacy of hippocampal stimulation to that of surgical treatments [13]. However, the cellular mechanism responsible for the effects of DBS remains largely undefined [14].
It is known that γ-aminobutyric acid (GABA) type A (GABAA) receptors play important roles in the development of epilepsy. The abundance of GABAA receptors is decreased in brain of patients with pharmacoresistant epilepsy. Experimental studies have obtained results to this effect [15]. Loss-of-function mutations in GABAA receptor genes are an important mechanism in the pathophysiology of familial idiopathic generalized epilepsy. Alterations in GABAA receptor subtypes may be involved in resistance to AEDs [16]. Neurodegeneration and associated GABAA receptor changes in the dentate gyrus are critically involved in the mechanisms underlying refractoriness of seizures in TLE [17].
Studies suggested that the GABAA receptor might be involved in the effect of hippocampal stimulation [18-21]. Recent publications have considered that the mechanism of hippocampal stimulation may be related to the extrasynaptic expression level of the GABAA receptor α5 subunit [22]. Our previously published studies have suggested that the GABAA receptor abundance is increased after Hip-LFS [23]. However, the mechanism underlying the increase of GABAA receptor abundance after Hip-LFS remains incompletely understood.
Cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) has been demonstrated to be closely related to epilepsy [24]. Epileptic brain regions prone to seizure activity exhibit persistent activation of CREB [24], and increased expression of phosphorylated CREB (p-CREB) has been observed in epileptic rats and patients with medically intractable epilepsy [25,26]. Mice with decreased CREB levels exhibit an approximately 50% reduction in spontaneous seizures following pilocarpineinduced status epilepticus and require greater stimulation to electrically kindle seizures [25]. Little is known about the changes in CREB and the relationship between CREB and GABAA receptors after Hip-LFS.
The gene encoding GABAA subunit α1 contains the cAMP response element (CRE) in its promoter [27]. Moreover, the genes encoding GABA receptors are targets of cAMP-response element-binding protein (CREB) modulation [3,28], and the surface expression of GABAA receptors is transcriptionally controlled by a system of interactions involving CREB [29]. It was reasonable to speculate that the Hip- LFS-induced increase in GABAA receptors in pharmacoresistant epileptic rats was associated with the decrease in CREB.
The present study aimed to observe the effects of Hip-LFS on GABAA receptors and CREB, as well as the relationship between GABAA receptors and CREB in pharmacoresistant epileptic rats.
Materials and Methods
▪ Materials
Subjects: This study was approved by the Animal Care and Use Committee of Guizhou Medical University. A total of 100 healthy adult male SD rats ranging from 50 to 60 days of age (provided by the Experimental Animal Centre of Guizhou Medical University) were used as subjects in the experiment. The rats weighed 200-250 g. They were individually housed in galvanized wire mesh cages with free access to food and water. The laboratory room temperature ranged from 21 to 25°C, and there was natural lighting.
Reagents: The reagents used in this study included the following: phenytoin sodium injection (PHT) and phenobarbital (PB, Shanghai New Asiatic Pharmaceuticals Co., Ltd, Shanghai, China), 10% chloral hydrate (Qilu Hospital of Shandong University), sodium chloride for injection (Guizhou Medical University), distilled water (Guizhou Medical University), penicillin sodium for injection (Shandong Lukang Pharmaceutical Co., Ltd, Jinan, China), self-curing denture acrylic (Shanghai Dental Material Factory, Shanghai, China), 502 superglue (Zhejiang Golden Roc Chemical Co., Ltd, Hangzhou, China), 4% paraformaldehyde/0.1 M phosphate buffer (pH 7.4, Wuhan Boster Biological Technology Co., Ltd, Wuhan China), anti-CREB antibody (E306, Abcam, Massachusetts, USA), anti-GABAA receptor α1 antibody (S95-35, Abcam company, Massachusetts, USA), phospho-CREB (Ser133; Cell Signaling Technology, Boston, USA), and PV-6001 (Beijing Zhongshan Golden-bridge Biological Technology Co., Ltd, Beijing, China).
Apparatus: The experimental apparatus consisted of a BL-420 biological functional system (Chengdu Technology & Market Co., Ltd), a Jiangwan Type I stereotaxic apparatus (Shanghai Chuansha Huamu Agricultural Machinery Factory), a P-22 dual-expanded metal mesh detachable shielded room (Changzhou No. 2 Radio Factory), enamelled nichrome wire (Chongqing Instrument and Meters Factory), a CH2510 three-hole wire connector (Ningbo Instrument and Meters Factory), a DK-8 D-type electrical thermostatic sink (Shanghai Senxin Experiment Instrument Co., Ltd), a DYY-8 voltage-type stabilized electrophoresis apparatus (Shanghai Qi’s Analysis Instrument Co., Ltd), a YXJ-2 centrifuge (Hunan Instrument Centrifuge Instrument Co., Ltd), an H6-1 miniature electrophoresis slot (Shanghai Lean Organic Glass Instrument Plant), a gel imaging system (Gene Genius), a U-3010 UV-Vis spectrophotometer (Hitachi), a liquid-removing device (range 100-1000 ml, 20-200 ml, 0.5- 10 ml) (Canada BBI), and the primer design software Primer Premier 5.0.
▪ Experimental materials
(1) Dental cement was prepared by mixing 502 superglue with liquid denture acrylic.
(2) The stimulating electrodes were bipolar electrodes (Dia. 0.1 mm with tip separation of 0.025 mm) made from enamelled nichrome wire.
Methods
▪Model Preparation
The method used to prepare the pharmacoresistant model of epilepsy was the same as the one described in our previously published studies. Briefly, the animals were anaesthetized with an intraperitoneal injection of 10% chloral hydrate (0.3 ml/100 g) and placed in a stereotaxic apparatus. Burr holes were drilled in the skull, and a bipolar enamelled nichrome electrode was implanted in the right basolateral amygdala. After implantation of the electrode, the rats were returned to their home cages. The rats were allowed to recover from surgery for at least seven days before being subjected to the experiment.
Electrical stimulations were delivered daily to the amygdala, starting 7 days after electrode implantation. The initial current intensity was 0.02 mA, with a frequency of 60 Hz and pulse duration of 1.0 ms. Train stimulation with a 0.05 ms delay was administered with an interval of 15.65 ms between pulses within the train, and the length of the stimulation train was 160 pulses. The amygdala after-discharge threshold (ADT) was recorded after each stimulation. The rats were stimulated at intervals of 5 min, and the current intensity was increased by 20% each time until discharges lasting for 3 seconds (or longer) were observed. The current intensity that produced the discharges mentioned above was considered the ADT [30].
Ten minutes after the ADT was determined, the rats were subjected to a kindling procedure [30]. The parameters for the kindling stimulation were as follows: the frequency was 60 Hz, the pulse duration was 0.1 ms, and the current intensity was 0.4 mA [31]. The kindling stimulation was administered once per day. The duration of the stimulation was twenty minutes [32]. Following each stimulus, electroencephalograms (EEGs) and seizure degree were recorded. Behaviour was continuously monitored during electrical stimulation and for several hours thereafter. The kindled seizures were considered successful when an after-discharge appeared on the amygdala EEG and three consecutive stage 5 seizures were observed in the rats.
Behavioural seizure stages as classified by Racine [33] were used to characterize behavioural seizure severity during daily kindling.
▪ Selection of pharmacoresistant rats
Control ADT measurement: Normal saline (the same volume as the antiepileptic drug to be injected) was injected into the abdominal cavity of each kindled epileptic rat, and the ADT was determined one hour later. The initial stimulus intensity was 20% of the post-kindled ADT, and the test stimulus was increased by 20% each time. The procedures for measurement of the control ADT were completed within 30-60 min.
ADT measurement after AEDs: To test for drug resistance or sensitivity, we compared ADT following administration of either saline or AEDs. If ADT was increased by 20% after intraperitoneal injection of AEDs compared with the control for at least three tests, the rats were considered pharmacosensitive. However, if ADT after intraperitoneal injection of AEDs did not increase or increased by less than 20% compared with the control, the rats were considered pharmacoresistant.
PHT (75 mg/kg) was administered by intraperitoneal injection 24 hours after the control ADT was determined. One hour after intraperitoneal injection of PHT, ADT was determined. The same procedures as the control ADT measurement were applied, and the control ADT was used as baseline. The current intensity producing amygdala potentials was determined as the ADT value after administration of the AEDs. The process of measurement was completed within 30-60 min. Drugs (PHT) were administered at least three times with an interval of one week, and the entire procedure took three to five weeks.
When the epileptic rats were found to be pharmacoresistant to PHT, the process of selection was continued using PB. PB (30 mg/kg) was administered by intraperitoneal injection, and the above procedures were repeated [34].
The anticonvulsant effects of PHE or PB were determined based on Loscher’s evaluation method [35]. Pharmacosensitive epileptic rats were defined as those in which consistent anticonvulsant effects (the ADT increased more than 20% for at least three times) were obtained after PHE or PB. Pharmacoresistant epileptic rats were defined as those that showed no anticonvulsant response (the ADT increased less than 20% or remained unchanged three times) to PHE or PB. Variable responders were defined as rats in which variable responses to PHE or PB (the ADT increased more than 20% at one time and less than 20% at another time) were obtained after drug administration. If the rats showed consecutive responses to the PHT or no responses to the PHT for three consecutive trials, the process of drug selection was terminated. If the rats showed variable responses to the PHT, the test increased to at most five iterations.
Experimental Grouping: Among the 100 SD rats, 10 of them were selected as normal controls. The other 90 rats were subjected to the kindling experiments to generate an epileptic model. Seven rats died unexpectedly, and the electrode fell off 5 rats during the kindling experiments. Finally, 78 rats underwent the kindling process, and 50 rats were successfully kindled.
The 50 kindled rats were subjected to pharmacoresistance selection. Six weeks after intraperitoneal injection of PHT and PB, twenty-three of the 50 epileptic rats were found to be resistant to PHT, but 7 out of them were sensitive to PB. Finally, 16 epileptic rats were found to be pharmacoresistant to both PHT and PB. The 16 pharmacoresistant epileptic rats were equally divided between a pharmacoresistant control group (PRC group, 8 rats) and a pharmacoresistant stimulation group (PRS group, 8 rats). The same number of pharmacosensitive epileptic rats were used as a pharmacosensitive control group (PSC group, 8 rats), and a normal rat control (NRC) group (8 rats) was also used.
Hippocampal Stimulation: An electrode was implanted into the right hippocampus of each rat in each of the four groups. The stereotaxic coordinates were as follows: 3.6 mm posterior to the bregmatic fontanel, ±2.4 mm lateral to the midline, 3.0 mm ventral to the skull surface. Hip-LFS was performed only in the PRS group seven days after the hippocampal electrode implantation. The hippocampal electrode was connected to the BL-420 Biological Functional System. Stimulation lasted 15 min per trial, and each stimulation consisted of a 1 Hz frequency pulse train, with each pulse having a duration of 1 ms and a current intensity of 100 μA(7). A 0.05 ms delay preceded the train. The rats were stimulated twice per day (9:00 in the morning and 9:00 in the evening) for two consecutive weeks. The other three groups received sham stimulation. Pulse trains at the same frequency as the PRS group were administered to the sham stimulation groups without connecting the hippocampal electrode to the instrument.
Sample Collection: Chloral hydrate (10%, 0.4 ml/100 g) was injected into the abdominal cavity of all the rats. The rats were then placed on their back on a dissection board, and the heart was exposed. The cardiac apex was localized, and a blunt puncture needle was inserted from the left ventricle to the aorta ascendens under direct vision. The right atrial appendage was then cut to allow blood flow out of the heart. The rats were perfused with 100 ml of cold normal saline through the heart, followed by 500 ml of 4% paraformaldehyde in 0.1 mol/L phosphate buffer solution. The total perfusion time was 1 hour. The hippocampus was removed from the skull and placed into the perfusate solution overnight at 4°C. The hippocampus was cut into two parts. One part was used for Western blotting, and the other was placed in a 20% sucrose solution for 12 hours in preparation for histochemical staining.
Western blot for measurements: The hippocampal samples were homogenized in a buffer containing Tris-HCl, NaCl, Triton X-100 and double-distilled water using a homogenizer. The mixture was centrifuged at 12000 rpm for 15 min at 4°C to isolate the supernatant. After protein quantification, the samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDSPAGE) and transferred onto a membrane made of polyvinylidene difluoride (PVDF). The membrane was blocked with blocking buffer overnight at 4°C and incubated the next day with anti-GABAAR α1, anti-CREB and antip- CREB antibodies (1:1000) for one hour at room temperature, followed by incubation with secondary antibody (1:5000). Finally, immunoreactive bands were visualized on film after incubation in a chemiluminescence substrate. GAPDH was used as an internal reference to normalize the results and was probed with the same procedures as GABAAR α1, CREB and p-CREB. Densitometric analysis of the bands was conducted using an image analysis system for Windows (National Institutes of Health, USA).
Immunohistochemical Observation: The hippocampal CA1 and CA3 areas were used for immunohistochemical analysis. Paraffin sections were routinely deparaffinized in water, then placed into 0.01 M citrate buffer and heated in a microwave for ten minutes. A 50 µl drop of 0.1% Triton was applied to each section, followed by incubation at 37°C for ten minutes. The sections were then flushed with phosphate-buffered saline (PBS) three times for three minutes each, then a drop of animal serum was applied for blocking, and the sections were incubated at 37°C for 15 min. A drop of rabbit anti-GABAA receptor subunit α1, anti-CREB or anti-p-CREB (1:100 dilutions) was applied to the section and incubated overnight at 4°C. The sections were then flushed with PBS three times for five minutes each, then a drop of biotin was applied to mark goat anti-or rabbit-IgG and incubated at 37°C for 20 min. The sections were again flushed three times with PBS for three minutes each, incubated at 37°C for 20 min in streptavidin peroxidase, and flushed again with three rinses of PBS, each lasting three minutes. Finally, the 3,3’-diaminobenzidine colour reaction, an alcohol dehydration series, and dimethyl benzene clearing were performed, and Permount was used to seal the slides.
After the hippocampal tissue sections were stained, images of the immunohistochemical staining were collected using a SONY camera and entered into the Biomias2001 image analysis system. Five areas (the size of the visual field size = 0.49296 mm2) on each slice were randomly selected to determine the average greyscale values of the positive neurons.
The greyscale value means the transmittance of the light. It represents the material content to be determined when image analysis was performed. The greater the greyscale values, the lower the material content. Greyscale value ranges from 0 to 255. In immunohistochemical analysis, the integrated optical density (IOD) is commonly used [36].The greyscale value is inversely proportional to the integrated optical density value. When the grey value is equal to 225, the IOD value is 0. The criteria for positive neurons were the appearance of brown-yellow granules in the cytoplasm.
Statistical Analysis
The experimental data were expressed as the mean ± standard deviation (X±s). All data were analysed using SPSS 11.5. Comparisons between two sample means were made using a t-test. One-way analysis of variance was used to make comparisons among multiple groups. A p-value of less than 0.05 was considered statistically significant. Statistical analysis was performed in consultation with the Department of Biostatistics of Guizhou Medical University.
Results
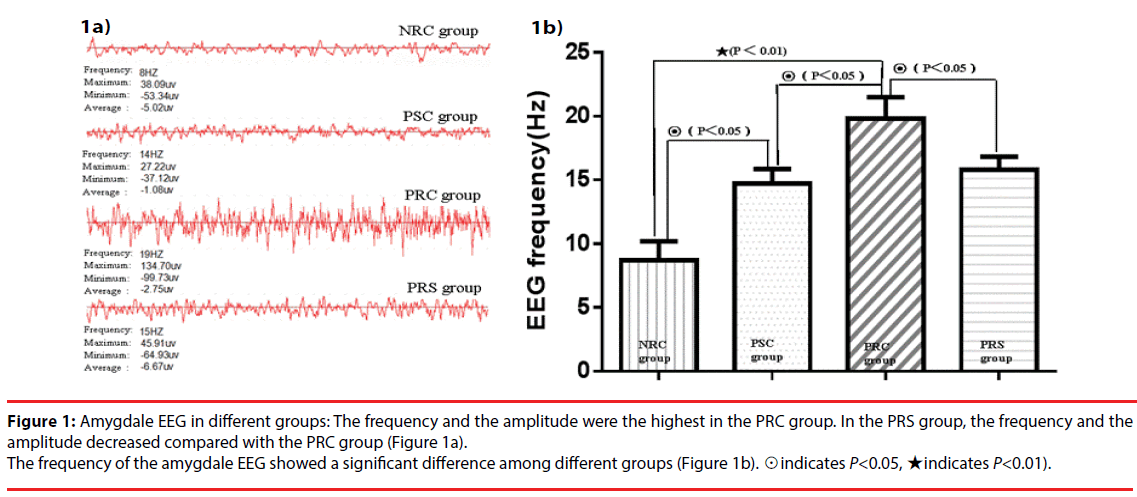
Changes in amygdala EEG
The frequency of the amygdala EEG increased remarkably in the PSC group (14.700±1.160 Hz) compared with the NRC group (8.7±1.49 Hz). It was the highest in the PRC group. The PRC group also displayed increased amplitude compared with the other groups. However, the frequency of amygdala EEG in the PRS group decreased remarkably compared with the PRC group. A significant difference in the EEG frequency was observed among the four groups (F=144.202, P<0.05). These results suggested that the pharmacoresistant epileptic rats showed higher frequency and amplitude of EEG activity than the pharmacosensitive ones (Figure 1). Hip- LFS decreased the amygdala EEG frequency and amplitude of the pharmacoresistant epileptic rats.
Figure 1: Amygdale EEG in different groups: The frequency and the amplitude were the highest in the PRC group. In the PRS group, the frequency and the amplitude decreased compared with the PRC group (Figure 1a).
The frequency of the amygdale EEG showed a significant difference among different groups (Figure 1b). ☉indicates P<0.05, ★indicates P<0.01).
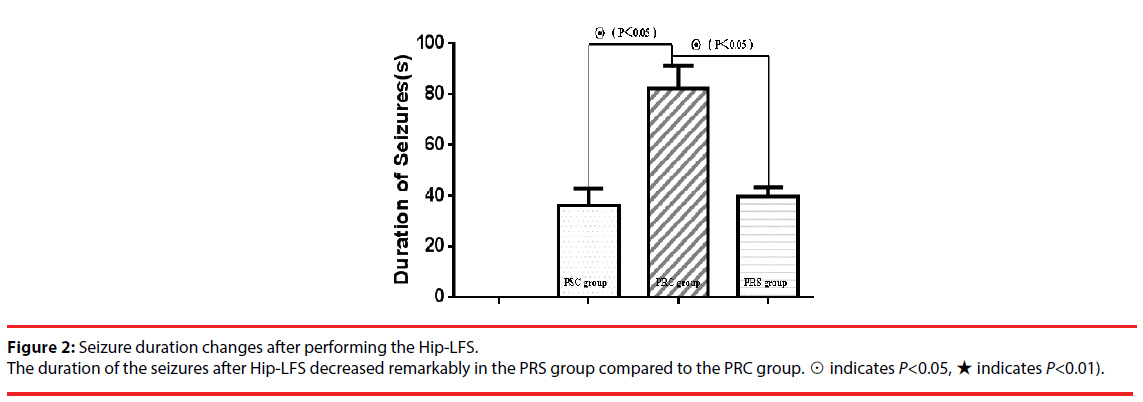
▪ Changes in epileptic seizures
After Hip-LFS for two weeks consecutively, the same current intensity used for kindling the rat was administered to induce epileptic seizures. The results showed that the Racine stages in the PRS group decreased compared with those in the PRC group (X2=20, P=0.000). The duration of the seizures in the PRS group (37.300±6.557) also reduced compared with those in the PRC group (82.100±9.158) to a statistically significant degree (Figure 2, P<0.05). These results suggested that the Hip-LFS displayed an inhibitory effect on seizures in the pharmacoresistant rats (Figure 3).
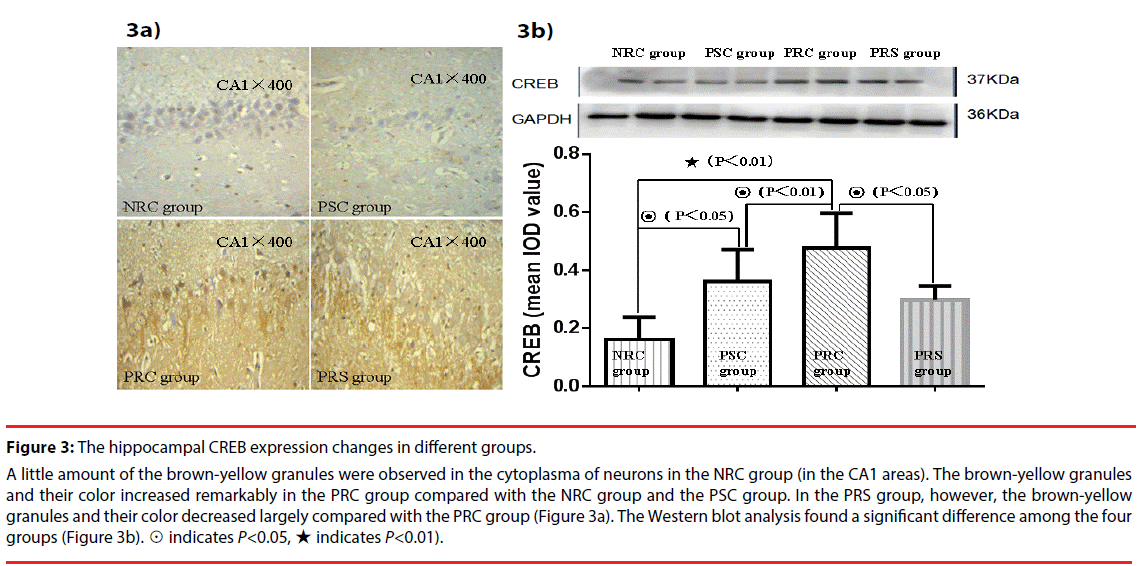
Figure 3: The hippocampal CREB expression changes in different groups.
A little amount of the brown-yellow granules were observed in the cytoplasma of neurons in the NRC group (in the CA1 areas). The brown-yellow granules and their color increased remarkably in the PRC group compared with the NRC group and the PSC group. In the PRS group, however, the brown-yellow granules and their color decreased largely compared with the PRC group (Figure 3a). The Western blot analysis found a significant difference among the four groups (Figure 3b). ☉ indicates P<0.05, ★ indicates P<0.01).
▪ Changes in CREB and p-CREB
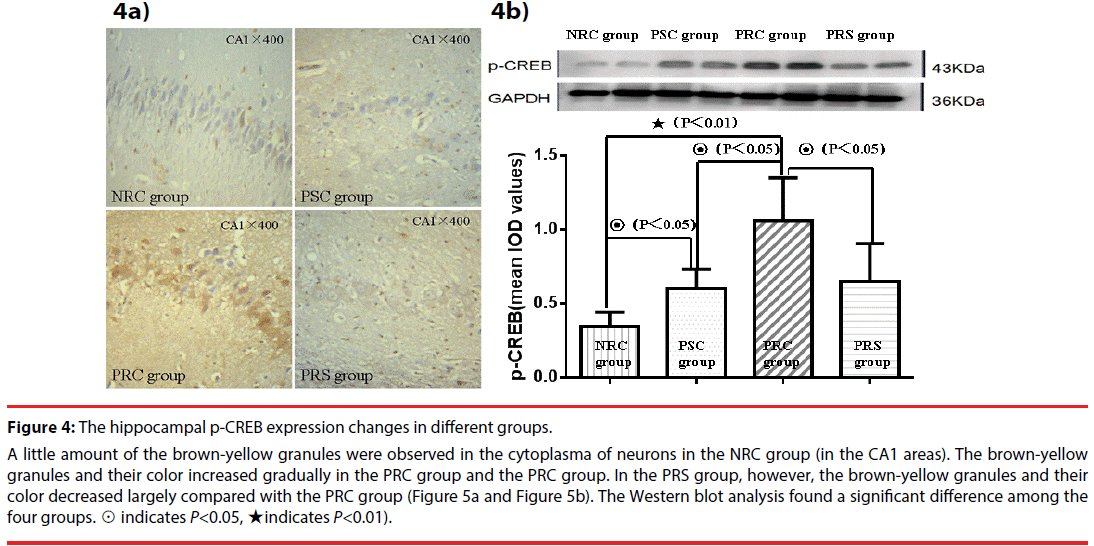
The CREB levels increased remarkably in the PRC group compared with the PSC group and the NRC group. A large number of brownyellow granules were observed in the cytoplasm of hippocampal neurons in the PRC group (Figure 4a). However, the CREB expression decreased significantly after two weeks of Hip-LFS. A significant difference in CREB abundance, as measured by Western blotting, was observed among the four groups (F=13.559, P<0.05). The amount of the CREB increased remarkably in the PSC group compared with the NRC group (Figure 4b). It was higher in the PRC group than in any of the other three groups. In the PRS group, however, CREB decreased remarkably after Hip-LFS compared with the PRC group. p-CREB displayed similar immunohistochemical results. The Western blot method also demonstrated a significant difference among the four groups (Figure 5a-b, F=20.018, P<0.05).
Figure 4: The hippocampal p-CREB expression changes in different groups.
A little amount of the brown-yellow granules were observed in the cytoplasma of neurons in the NRC group (in the CA1 areas). The brown-yellow granules and their color increased gradually in the PRC group and the PRC group. In the PRS group, however, the brown-yellow granules and their color decreased largely compared with the PRC group (Figure 5a and Figure 5b). The Western blot analysis found a significant difference among the four groups. ☉ indicates P<0.05, ★indicates P<0.01).
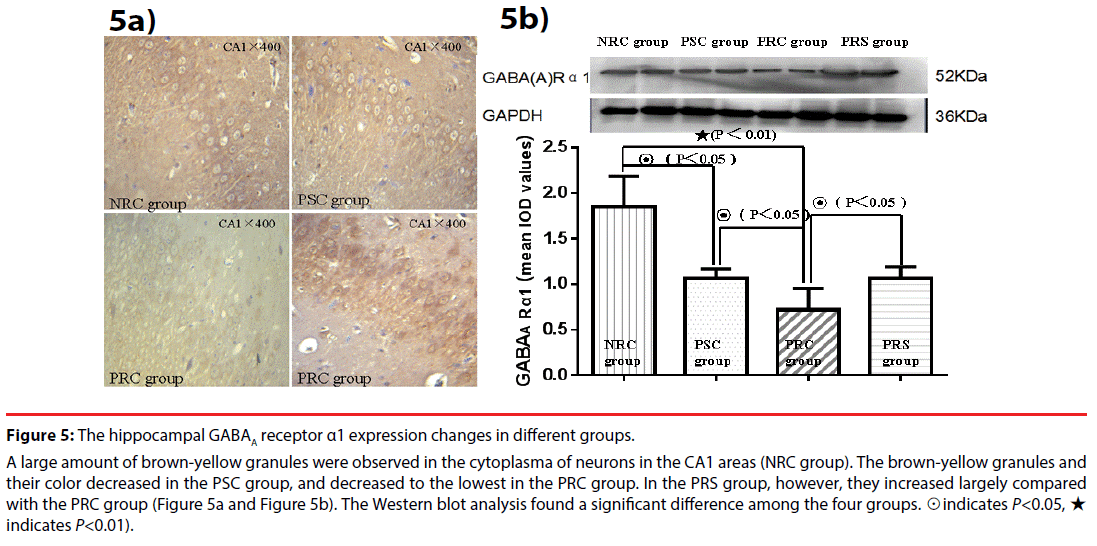
Figure 5: The hippocampal GABAA receptor α1 expression changes in different groups.
A large amount of brown-yellow granules were observed in the cytoplasma of neurons in the CA1 areas (NRC group). The brown-yellow granules and their color decreased in the PSC group, and decreased to the lowest in the PRC group. In the PRS group, however, they increased largely compared with the PRC group (Figure 5a and Figure 5b). The Western blot analysis found a significant difference among the four groups. ☉indicates P<0.05, ★ indicates P<0.01).
The ratio of p-CREB to total CREB in the PSC group was approximately 1.68. It increased to approximately 2.2 in the PRC group. After two weeks of Hip-LFS, both total CREB and p-CREB were decreased compared with the PRC group, and the ratio of p-CREB to total CREB was approximately 2.17.
These results suggested that increased CREB might be associated with the development of pharmacoresistant epilepsy and that the effect of the Hip-LFS might be achieved by decreasing CREB.
▪ Changes in GABAA receptor subunit α1
The NCR group showed a large number of brown-yellow granules in the cytoplasm of the hippocampal neurons. There were fewer brownyellow granules in the PSC group and the PRC group. The quantity was lowest in the PRC group. After Hip-LFS, the PRS group showed a large increase in the quantity of brownyellow granules compared with the PRC group (Figure 5a).
The Western blot demonstrated that a significant difference in GABAA α1 existed among the four groups (F=48.123, P<0.05). The amount of GABAA α1 decreased in the PSC group compared with the NRC group and was the lowest in the PRC group, suggesting that decreased GABAA α1 might be associated with the development of the pharmacoresistance. The abundance of GABAA receptor α1 was significantly higher in the PRS group than in the PRC group, indicating that its abundance increased after two weeks of Hip-LFS. These findings suggested that the Hip-LFS might promote the GABAA receptor subunit α1 expression in such a way that it plays a role in antiepileptic effects (Figure 5b).
Discussions
TLE is the most common form of partial epilepsy and is often associated with pharmacoresistance [37]. Although traditional surgery by selectively ablating the anterior lobe or the amygdalahippocampus could achieve favourable results, up to 30% of TLE cases are unsuitable for resective neurosurgery [3]. Clinical and experimental studies have demonstrated that Hip-LFS was effective in the treatment of intractable epilepsy [5-12,38,39]. Hip-LFS has been proposed as a possible clinic treatment for patients with mesial TLE [40]. In the present study, an amygdalakindled rat model of epilepsy was established and the pharmacoresistant epileptic rats were selected by their response to PHT and PB. Subsequently, the selected pharmacoresistant epileptic rats were used as subjects to investigate the effects of Hip- LFS. Hip-LFS displayed a remarkable decrease in the seizure degree and the frequency and amplitude of the amygdala EEG. Our previous studies have also demonstrated that Hip-LFS inhibited amygdala stimulus-induced epileptic seizures and after-discharges [5,6].
However, the mechanism underlying the effects of Hip-LFS in the treatment of pharmacoresistant TLE remains poorly understood. It is generally known that GABA plays an important role in the pathogenesis of intractable epilepsy. GABA interacts with three different types of receptors [41]. Previously published studies suggested that the GABAA receptor might be involved in the effects of Hip-LFS [18-21]. The GABAA receptors increased significantly following two weeks of Hip-LFS [23], and the gene that encodes them is a target of cAMP response element-binding protein (CREB) modulation [3,28]. Thus, it is reasonable to speculate that the increased GABAA receptor levels were correlated with the CREB changes during Hip-LFS in the treatment of the pharmacoresistant epileptic rats.
The present study demonstrated that the CREB and the phosphorylation CREB (p-CREB) increased remarkably, and the GABAA subunit α1 decreased in the pharmacoresistant epileptic rats compared with the control. /increased expression of p-CREB was observed in epilepsy rats and in the seizure onset region of humans with medically intractable epilepsy [25,26]. Epileptic brain regions prone to seizures showed persistent activation of CREB [24]. Impaired GABAergic inhibition within neuronal networks can lead to hypersynchronous firing patterns that are the typical cellular hallmark of convulsive epileptic seizures [42]. These studies suggested that both CREB and GABAA receptors are closely associated with the development of epilepsy.
After performing the Hip-LFS for two weeks, the GABAA subunit α1 levels displayed a striking rise in the PRS group compared with the PRC group. However, CREB and p-CREB decreased significantly. Simultaneously, the degree and duration of seizures were decreased remarkably, as well as the amygdala EEG frequency. Mice with decreased CREB levels have a ~50% reduction in spontaneous seizures following pilocarpine-induced status epilepticus and require more stimulation to electrically kindle [25]. The gene encoding GABAA subunit α1 contains the cAMP response element in its promoter [27], and surface expression of GABAA receptors is transcriptionally controlled by a system of interactions involving CREB [29]. Our previously published studies have demonstrated that GABAA receptor abundance is negatively correlated with CREB levels [43]. Combined with these studies, our results suggested that Hip-LFS increases the level of GABAA subunit α1 and decreases the level of CREB. The increased abundance of GABAA subunit α1 after Hip-LFS might be associated with the decrease in CREB expression. Recently, studies have suggested that altering CREB activity might modify epileptogenensis [25]. Our experiments suggested that inhibiting CREB activity might provide a therapeutic strategy for pharmacoresistant epilepsy. The effects of Hip-LFS on pharmacoresistant epilepsy might be achieved partly by decreasing the amount of CREB and increasing the abundance of GABAA receptors.
However, we were unable to block the effects of CREB on the Hip-LFS-induced increase in GABAA receptors. Furthermore, spontaneous seizures in the epileptic rats were not monitored during all processes of the experiment. These may be limitations of the present study. Further studies are required to address the relationship between GABAA receptors and CREB in pharmacoresistant epileptic rats following Hip- LFS.
Conflict of interest
The authors declare that they have no any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work.
Source of Funding
This research was supported by the Natural Science Fund of China (81560222/H0913, 81241129/H0913) and Program for Changjiang Scholars and Innovative Research Team in University (IRT10358).
Acknowledgements
We are indebted to the Guizhou Centre of Disease Control and Prevention for providing us with the laboratory platform. We also wish to thank all the postgraduates involved for their hard work during the study.
References
- Pittau F, Vulliemoz S. Functional brain networks in epilepsy: recent advances in noninvasive mapping. Curr. Opin. Neurol 28(4), 338-343 (2015).
- Rosillo-de la Torre A, Luna-Barcenas G, Orozco-Suarez S, et al. Pharmacoresistant epilepsy and nanotechnology. Front. Biosci (Elite Ed) 6(1), 329-340 (2014).
- Ge Y, Hu W, Liu C, et al. Brain stimulation for treatment of refractory epilepsy. Chin. Med. J (Engl) 126(17), 3364-3370 (2013).
- Van Nieuwenhuyse B, Raedt R, Delbeke J, et al. In search of optimal DBS paradigms to treat epilepsy: bilateral versus unilateral hippocampal stimulation in a rat model for temporal lobe epilepsy. Brain. Stimul 8(2), 192-199 (2015).
- Wang L, Shi J, Wu G, et al. Hippocampal low-frequency stimulation increased SV2A expression and inhibited the seizure degree in pharmacoresistant amygdala-kindling epileptic rats. Epilepsy. Res 108(9), 1483-1491 (2014).
- Wu G, Hong Z, Li Y, et al. Effects of low-frequency hippocampal stimulation on gamma-amino butyric acid type B receptor expression in pharmacoresistant amygdaloid kindling epileptic rats. Neuromodulation 16(2), 105-113 (2013).
- Zhang SH, Sun HL, Fang Q, et al. Low-frequency stimulation of the hippocampal CA3 subfield is anti-epileptogenic and anti-ictogenic in rat amygdaloid kindling model of epilepsy. Neurosci. Lett 455(1), 51-55 (2009).
- Tellez-Zenteno JF, Wiebe S. Hippocampal stimulation in the treatment of epilepsy. Neurosurg. Clin. N. Am 22(4), 465-475 (2011).
- Rashid S, Pho G, Czigler M, et al. Low frequency stimulation of ventral hippocampal commissures reduces seizures in a rat model of chronic temporal lobe epilepsy. Epilepsia 53(1), 147-156 (2012).
- McLachlan RS, Pigott S, Tellez-Zenteno JF, et al. Bilateral hippocampal stimulation for intractable temporal lobe epilepsy: impact on seizures and memory. Epilepsia 51(2), 304-307 (2010).
- Cukiert A, Cukiert CM, Burattini JA, et al. Seizure outcome after hippocampal deep brain stimulation in a prospective cohort of patients with refractory temporal lobe epilepsy. Seizure 23(1), 6-9 (2014).
- Akman T, Erken H, Acar G, et al. Effects of the hippocampal deep brain stimulation on cortical epileptic discharges in penicillin - induced epilepsy model in rats. Turk. Neurosurg 21(1), 1-5 (2011).
- Schulze-Bonhage A. Deep brain stimulation: a new approach to the treatment of epilepsy. Dutsch. Arztebl. Int 106(24), 407-412 (2009).
- Ghotbedin Z, Janahmadi M, Mirnajafi-Zadeh J, et al. Electrical low frequency stimulation of the kindling site preserves the electrophysiological properties of the rat hippocampal CA1 pyramidal neurons from the destructive effects of amygdala kindling: the basis for a possible promising epilepsy therapy. Brain. Stimul 6(4), 515-523 (2013).
- Wu guofeng Sj, Hong zhen, Zhou feng. Dereased expressions of GABA receptors in the hippocampal tissues of pharmacoresistant temporal lobe epileptic rats. Chin. J. Neurol 46(1), 1-5 (2013).
- Bethmann K, Fritschy JM, Brandt C, et al. Antiepileptic drug resistant rats differ from drug responsive rats in GABAA receptor subunit expression in a model of temporal lobe epilepsy. Neurobiol. Dis 31(2), 169-187 (2008).
- Volk HA, Arabadzisz D, Fritschy JM, et al. Antiepileptic drug-resistant rats differ from drug-responsive rats in hippocampal neurodegeneration and GABAA receptor ligand binding in a model of temporal lobe epilepsy. Neurobiol. Dis 21(3), 633-646 (2006).
- Velasco M, Velasco F, Velasco AL, et al. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia 41(2), 158-169 (2000).
- Velasco AL, Boleaga B, Brito F, et al. Absolute and relative predictor values of some non-invasive and invasive studies for the outcome of anterior temporal lobectomy. Arch. Med. Res 31(1), 62-74 (2000).
- Velasco M, Velasco F, Velasco AL. Centromedian-thalamic and hippocampal electrical stimulation for the control of intractable epileptic seizures. J. Clin. Neurophysiol 18(6), 495-513 (2001).
- Cuellar-Herrera M, Velasco M, Velasco F, et al. Evaluation of GABA system and cell damage in parahippocampus of patients with temporal lobe epilepsy showing antiepileptic effects after subacute electrical stimulation. Epilepsia 45(5), 459-466 (2004).
- Shen FZ, Wang F, Yang GM, et al. The effects of low-frequency electric stimulus on hippocampal of Effects of low-frequency electric stimulus on hippocampal of alpha5 subunit of extra synapse GABAA receptor in kainic acid-induced epilepsy rats. Zhonghua. Yi. Xue. Za. Zhi 93(1), 550-553 (2013).
- Wu G, Wang L, Hong Z, et al. Hippocampal low-frequency stimulation inhibits afterdischarge and increases GABA (A) receptor expression in amygdala-kindled pharmacoresistant epileptic rats. Neurol. Res 39(8), 733-743 (2016).
- Beaumont TL, Yao B, Shah A, et al. Layer-specific CREB target gene induction in human neocortical epilepsy. J. Neurosci 32(41), 14389-14401 (2012).
- Zhu X, Han X, Blendy JA, et al. Decreased CREB levels suppress epilepsy. Neurobiol. Dis 45(1), 253-263 (2012).
- Guo J, Wang H, Wang Q, et al. Expression of p-CREB and activity-dependent miR-132 in temporal lobe epilepsy. Int. J. Clin. Exp. Med 7(5), 1297-1306 (2014).
- Steiger JL, Russek SJ. GABAA receptors: building the bridge between subunit mRNAs, their promoters, and cognate transcription factors. Pharmacol. Ther 101(3), 259-281 (2004).
- Impey S, McCorkle SR, Cha-Molstad H, et al. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119(7), 1041-1054 (2004).
- Hu Y, Lund IV, Gravielle MC, et al. Surface expression of GABAA receptors is transcriptionally controlled by the interplay of cAMP-response element-binding protein and its binding partner inducible cAMP early repressor. J. Biol. Chem 283(14), 9328-9340 (2008).
- Freeman FG, Jarvis MF. The effect of interstimulation interval on the assessment and stability of kindled seizure thresholds. Brain. Res. Bull 7(6), 629-633 (1981).
- Lopez-Meraz ML, Neri-Bazan L, Rocha L. Low frequency stimulation modifies receptor binding in rat brain. Epilepsy. Res 59(2-3), 95-105 (2004).
- Nissinen J, Halonen T, Koivisto E, et al. A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy. Res 38(2-3), 177-205 (2000).
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol 32(3), 281-294 (1972).
- Brandt C, Bethmann K, Gastens AM, et al. The multidrug transporter hypothesis of drug resistance in epilepsy: Proof-of-principle in a rat model of temporal lobe epilepsy. Neurobiol. Dis 24(1), 202-211 (2006).
- Loscher W, Reissmuller E, Ebert U. Anticonvulsant efficacy of gabapentin and levetiracetam in phenytoin-resistant kindled rats. Epilepsy. Res 40(1), 63-77 (2000).
- Yi J, Mao X, Chen L, et al. Illuminant direction estimation for a single image based on local region complexity analysis and average gray value. Appl. Opt 53(2), 226-236 (2014).
- de Moura JC, Tirapelli DP, Neder L, et al. Amygdala gene expression of NMDA and GABAA receptors in patients with mesial temporal lobe epilepsy. Hippocampus 22(1), 92-97 (2012).
- Rashid S, Pho G, Czigler M, et al. Low frequency stimulation of ventral hippocampal commissures reduces seizures in a rat model of chronic temporal lobe epilepsy. Epilepsia 53(1), 147-156 (2011).
- Vonck K, Sprengers M, Carrette E, et al. A decade of experience with deep brain stimulation for patients with refractory medial temporal lobe epilepsy. Int. J. Neural. Syst 23(1), 1250034 (2013).
- Tellez-Zenteno JF, McLachlan RS, Parrent A, et al. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology 66(10), 1490-1494 (2006).
- Czuczwar SJ, Patsalos PN. The new generation of GABA enhancers. Potential in the treatment of epilepsy. CNS. Drugs 15(5), 339-350 (2001).
- Errington AC, Cope DW, Crunelli V. Augmentation of Tonic GABAA Inhibition in Absence Epilepsy: Therapeutic Value of Inverse Agonists at Extrasynaptic GABAA Receptors. Adv. Pharmacol. Sci 790590 (2011).
- Jinpeng Yu ZL, Likun Wang, Guofeng Wu, et al. Increased GABA (A) Receptors α1, γ2, δ Subunits might be Associated with the Activation of the CREB Gene in Low Mg2+ Model of Epilepsy. Neuropsychiatry 7(4), 640-648 (2017).