Research Article - Neuropsychiatry (2017) Volume 7, Issue 2
Assessment of Concussion/Mild Traumatic Brain Injury-Related Fatigue, Alertness, and Daytime Sleepiness: A Diagnostic Modelling Study
- Corresponding Author:
- Tatyana Mollayeva, MD, PhD
Postdoctoral research fellow, Rehabilitation Science Institute
University of Toronto, Toronto Rehabilitation Institute
550 University Avenue, Rm 11207, Toronto, Ontario M5G 2A2, Canada
Tel: 416-597-3422 ext 7848
Fax: 416-946-8570
E-mail: [email protected]
Abstract
Objective:
Fatigue, alertness and daytime sleepiness are complex perceived states with significant implications for health and safety. These states are not diagnostically specific, and they may, or may not share interrelated features. Potential commonality or discordance is challenging in clinical situations, especially in controversial neurologic disorders, as in cases of concussion/ mild traumatic brain injury (mTBI).
Methods:
We performed a diagnostic multivariable modeling study to explore associations between patients’ characteristics, results of imaging tests and clinical investigations, and the states of fatigue, alertness and daytime sleepiness. The intensity of fatigue, alertness and daytime sleepiness was measured using the standardized Fatigue Severity Scale (FSS), the Toronto Hospital Alertness Test (THAT), and the Epworth Sleepiness Scale (ESS). Univariate and multivariate linear regression models were used to explicate covariates of fatigue, alertness, and daytime sleepiness.
Results:
A total of 94 patients (45.20 ± 9.94 years; 61.2% males) with an established diagnosis of concussion/mTBI were included in the current analysis. Our results revealed that fatigue and alertness are associated with covariates within the domain of brain function integrity, and that daytime sleepiness is associated with cultural and physiological bodily states. In the final fully adjusted multivariable regression models, several covariates accounted for 57.2% of the fatigue variance, 41.2% of alertness variance, and 27.1% of the sleepiness variance. While fatigue and alertness share covariates, daytime sleepiness represents a distinct construct in persons with concussion/mTBI.
Conclusions:
Our findings challenge the commonly held view that fatigue, alertness and daytime sleepiness are perceived states on the same continuum. The implications of this finding have direct relevance to the clinical approach towards patients presenting with fatigue, impaired alertness or excessive daytime sleepiness after concussion/mTBI.
Keywords
Fatigue, Alertness, Daytime sleepiness, Traumatic brain injury, Concussion, Diagnostic modeling
Acronyms
BMI = Body Mass Index; DSM = Diagnostic and Statistical Manual of Mental Disorders; ESS = Epworth Sleepiness Scale; FSS = Fatigue severity scale; HADS-A = Hospital Anxiety and Depression Scale; ISI = Insomnia Severity Index; LOC = Loss of Consciousness; MRI = Magnetic Resonance Imaging; mTBI = Mild Traumatic Brain Injury; PHQ-9 = Patient Health Questionnaire- 9; PR = Patient-Reported; PTA = Post-Traumatic Amnesia; P-VAS = Pain Visual Analogue Scale; RDI = Respiratory Disturbance Index; RL=Restless Legs; RTW = Return to Work; SD=Standard Deviation; THAT = Toronto Hospital Alertness Test; TSI = Time Since Injury; TBI = Traumatic brain injury; TRIPOD = Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis; VIF = Variance Inflation Factor; UHN = University Health Network
Introduction
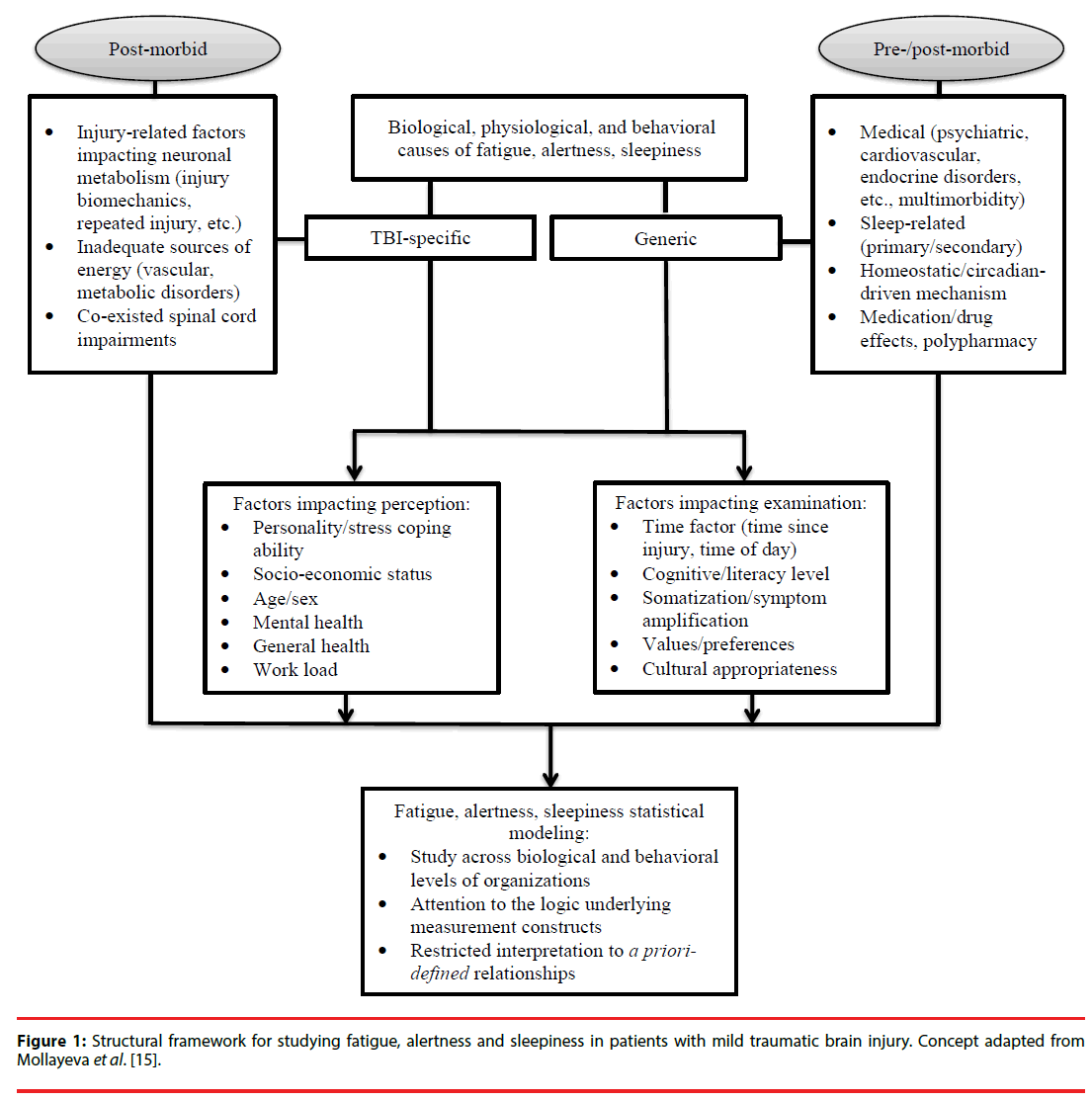
Fatigue, alertness and daytime sleepiness are complex perceived states with significant implications for health and safety [1-3]. The Stedman Medical Dictionary defines fatigue as, “(1) that state, following a period of mental or bodily activity, characterized by a lessened capacity for work and reduced efficiency of accomplishment, usually accompanied by a feeling of weariness, sleepiness, or irritability; may also supervene when, from any cause, energy expenditure outstrips restorative processes and may be confined to a single organ, and (2) sensation of boredom and lassitude due to absence of stimulation, monotony, or lack of interest in one’s surroundings”[4]. The same dictionary defines sleepiness as, “(1) drowsiness; an inclination to sleep, and (2) a condition of semiconsciousness approaching coma” [5]. ‘Alertness’ is not defined in the dictionary, but appears under ‘lethargy’, defined as, “a relatively mild impairment of consciousness causing reduced alertness and awareness; this condition has many causes but is ultimately due to generalized brain dysfunction”[4]. Clearly, the three terms, fatigue, alertness, and sleepiness are not diagnostically specific. They may or may not share interrelated features, and this makes it difficult to deal clinically with neurological disorders in which these symptoms are present. This is particularly relevant to the diagnostic uncertainty surrounding long-term neurological disorders that are difficult to explain – for example, when the injury has involved relatively limited force to the head and limited injury to the brain’s structure, as in concussion, a most common form of mild traumatic brain injury (mTBI) [5-7].
In the United States, the most widely accepted criteria for concussion/mTBI is proposed by the American Congress of Rehabilitation Medicine, which defines it as, “a physiological disruption of brain function as a result of a traumatic event as manifested by at least one of the following: Alteration of mental state, loss of consciousness (LOC), loss of memory or focal neurological deficits that may or may not be transient” [8].
Concussion/mTBI comprises more than 85% of all medically-treated TBIs, yet it remains among the most challenging and controversial of neurological disorders [9-11]. While many patients recover fully within days or weeks [12-14], it is estimated that at least 15% will experience delayed recovery, with disabling symptoms persisting beyond three months [15]. Many of these symptoms are not specific to TBI and are common in the general population, including fatigue, difficulty with attention/ maintaining alertness, and daytime sleepiness [16]. Despite great interest in the study of perceived states, it is unclear whether any of these states (fatigue, alertness, or daytime sleepiness) represents a physiological state or a composite symptom cluster that varies depending on the mechanism of the concussion/ mTBI, premorbid medical state, psychiatric morbidity, psychosocial issues, or is related to medication/drug effects. In other words, each of these states (i.e., fatigue, daytime sleepiness, or impaired alertness) could be overt expression of multiply-determined neurophysiological, psychological, and behavioral processes that contribute to a final common pathway of a negative perceived state post-injury (Figure l). Therefore, attention to potential pathways is key to determining how to manage post-concussive symptoms and to search for effective solutions. Furthermore, understanding how fatigue, alertness, and daytime sleepiness are related or distinct is important for a broader health and safety management system in concussion/mTBI, especially regarding release to return to work after injury (i.e., assessment of fitness for duty). The concurrent comparison of the factors associated with perceived fatigue, alertness, and daytime sleepiness will indirectly indicate whether these three constructs constitute a symptom cluster in concussion/mTBI or have different networks. Therefore, our aim was to study the three perceived states among patients experiencing delayed recovery from concussion/mTBI. On the basis of previous research into factors related to fatigue, alertness, and daytime sleepiness in TBI [17- 21], we hypothesized that: (1) fatigue and alertness are closely related constructs, and substantial overlap in factors associated with these states would be observed; (2) daytime sleepiness in concussion/mTBI is a distinct (from fatigue and alertness) construct; and (3) fatigue, alertness, and daytime sleepiness have a sleep-related component as a shared factor.
Material and Methods
The study protocol was approved by ethics committees at clinical and academic institutions with which the authors were affiliated. The study complied with the principle of the Declaration of Helsinki. To explicate the physiological, pathological and behavioral processes of fatigue, alertness, and daytime sleepiness in concussion/ mTBI, we grouped elements across simpler organizational themes that allowed us to begin with associations, followed by statistical models at various hierarchical levels of aggregation. We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines [22].
▪ Procedure and participants
The Neurology Service of the largest rehabilitation teaching hospital in Canada has a contractual agreement with the insurer (i.e., Workers Safety and Insurance Board (WSIB)) to provide expert diagnostic opinions for persons who have or are suspected to have sustained neurological injuries at work. Injured persons were recruited at the admission to the insurer’s clinic. Initial contact was made with 178 subjects, of whom 110 provided written consent to participate and completed the required assessments. At the same time participants underwent comprehensive specialty investigations (i.e., psychiatry, neurology, occupational therapy, physiotherapy, and neuropsychology) and neuroimaging testing for establishing TBI diagnoses. The researchers were blinded to the participant’s diagnosis until their medical charts became available for review, after clinical assessments were complete. All participants were also asked for consent to access their pre-morbid clinical and insurer’s files, and all gave written permission. To assess our sample’s representativeness indirectly, we compared it to a consecutive sample of persons (n = 294) who were referred and assessed in the same clinic during 2003 [23]. No significant differences were observed in injury severity, sex, age, or clinical diagnosis. To maintain sample homogeneity in terms of injury severity, we used data for persons (n = 94) with an established diagnosis of concussion/ mTBI (Appendix A).
▪ Instruments and measures
Standardized patient-reported (PR) measures based on previous work as most suited to persons with TBI [16,21] were used to assess each participant’s opinion and appraisal of his/ her levels of fatigue, alertness, and sleepiness. Additional PR measures covered sleep-, clinical-, injury- related, and behavioral variables pertinent to our hypotheses [16,21]. Medical files provided data on brain injury mechanism, presence of LOC, post-traumatic amnesia (PTA), neuroimaging findings, and clinical diagnosis pre- and post-injury, including the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV-TR [24]. Injury-related variables were also collected from the participants’ WSIB files, and included working status at the time of investigation, previous work-related injuries (if any), employer-patient relations, and insurerpatient relations Measurements by clinicians (i.e., all diagnostic investigations and clinical assessments) and those obtained directly from the participants (i.e., all PRs) were collected within a short period during which no treatment was commenced. Detailed descriptions of the studied independent variables are presented in Appendix B.
The primary outcome variables were level of fatigue, alertness and sleepiness as determined by the Fatigue Severity Scale (FSS) [25], Toronto Hospital Alertness Test (THAT) [26] and Epworth Sleepiness Scale (ESS) [27]. For information on the psychometric properties of the PR measures used, we refer the reader to published studies [16,21].
▪ Statistical Analysis
SAS software (version 9.3, SAS Inc., Cary, NC) was used for all data analyses.
Means and standard deviations or medians and ranges were used for continuous data and frequency counts for categorical data. The FSS, THAT and ESS scores were skewed in our sample; however, their residuals (a more important ‘test’ for normality) were distributed normally [28]. To detect potential collinearity in the modeling process, we used Spearman’s correlation coefficients for all a priori-defined associations between continuous variables and one-way analysis of variance for categorical/ binary explanatory variables with two or more levels. To assess the inflation of variances of the estimated coefficients, we used the variance inflation factor (VIF); a VIF > 6 was considered to indicate collinearity [29]. Because one of our independent variables (i.e., measure of depression by the Patient Health Questionnaire (PHQ)- features the item ‘feeling tired or having little energy’, which is arguably related to the constructs under study (i.e., fatigue, alertness, and sleepiness), we removed this item from the total PHQ-9 score when reporting associations. We applied stepwise multiple linear regressions with elimination to build models for each category of variables, grouped by (1) socio-demographic; (2) brain-injury related; (3) medical; (4) sleeprelated (including circadian-driven mechanism); and (5) medication/substance effects.
All hypothesized variables associated with outcomes of interest at statistically significant level p≤.2, and those consistently reported in the literature being associated with constructs under the study, were included initially; all variables identified as significant at p ≤ .1 were included in the final model. Sex, age, and time of assessment were included in every model that used PR measures. Several items within the PR measures (~5%) were not completed by participants. We examined missing items and did not observe a relationship with responses on other items within these PR measures. Therefore, we treated them as missing at random, and used single data imputation to estimate the values of missing items [30]. Our sample size was sufficient to permit accurate estimation of regression coefficients, standard errors, and confidence intervals in all linear regression models [31].
▪ Power calculation
This study (see study context section) recruited 110 persons with TBI, of which 94 participants were diagnosed with mTBI/concussion. A published series of Monte Carlo simulation studies suggest that linear regression models will produce accurate (i.e., relative bias less than 10%) estimation of regression coefficients, standard errors, and confidence intervals with a sample size of at least two subjects per variable [31]. The present study two or more ensured the recommended number of participants per independent variable and, therefore, secured sufficient power in each multivariable model.
To eliminate the potential experiment-wise error, we conducted 1) preliminary analyses (i.e., examined descriptive statistics of the continuous variables, checked the normality assumption by examining histograms of the continuous variables, checked the linearity assumption by examining correlations between continuous variables and scatter diagrams of the independent variable versus each dependent variable, we ran/ analyzed ANOVA to reduce probability of Type 1 error for categorical/binary explanatory variables with two or more levels); 2) multiple linear regression analysis utilizing collinearity diagnostics to check for multicollinearity, examined residual plots to check error variance assumptions, examined influence diagnostics (residuals, beta degree of freedom) to check for outliers; and 3) final regression models were fit with variables detected in individual, rigorously investigated models.
Results
Table 1a,b present the characteristics of the 94 participants (45.20 ± 9.94 years; 61.2% males) with a diagnosis of concussion/ mTBI, established upon completion of clinical investigations. Seventy-four (78%) were born in Canada, 69 (73%) were single/widowed or divorced, and 56 (60%) had post-secondary degrees. Median time since injury (TSI) was 197 days [interquartile range, 139-416]. The major mechanisms of injury were falls (from elevation 19% and same level 18%), being struck by (19%) or against (17%) an object, motor vehicle incidents (13%), and being struck by a living subject (10.5%). Among persons with documented LOC and/or PTA, 31% had experienced LOC and 25% PTA. Previous head injuries were documented in the files of 23 persons (25%). Eighty-four participants underwent MRI or CT imaging; none exhibited trauma-related brain changes; scattered foci of hyper-intensity were detected in 27 participants (32%). Many of the participants were diagnosed with one or more DSM-IV TR disorders (Table 1a,b), including cognitive (63%), adjustment (51%), anxiety (45%) mood (42%), somatoform (29%), substance-related (15%), and sleep (10%) disorders; eight of the latter had sleep-related breathing disorders.
| Category | Variables | n (%N*) | FSS score mean (SD) | P-value | THAT score mean (SD) | P-value | ESS score mean (SD) | P-value |
|---|---|---|---|---|---|---|---|---|
| Socio-demographic, psychosocial | Sex | |||||||
| Male | 58 (62) | 43.50 (15.89) | 0.093 | 21.30 (9.22) | 0.169 | 8.53 (5.69) | 0.770 | |
| Female | 36 (38) | 48.81 (12.77) | 19.06 (8.88) | 8.89 (5.70) | ||||
| Born in Canada | ||||||||
| Yes | 75 (80) | 44.56 (15.25) | 0.211 | 20.66 (9.72) | 0.644 | 9.79 (5.48) | <0.001 | |
| No | 19 (20) | 49.37 (13.23) | 19.53 (8.73) | 4.26 (4.05) | ||||
| English first language | ||||||||
| Yes | 77 (82) | 44.84 (15.30) | 0.345 | 20.53 (9.40) | 0.838 | 9.35 (5.54) | 0.012 | |
| No | 17 (18) | 48.65 (13.05) | 20.00 (10.17) | 5.59 (5.50) | ||||
| Marital status | ||||||||
| Married/common law | 69 (73) | 45.25 (14.72) | 0.761 | 18.84 (9.95) | 0.330 | 8.90 (5.32) | 0.231 | |
| Single/divorced/widowed | 25 (27) | 46.32 (15.77) | 21.02 (9.33) | 8.21 (6.69) | ||||
| Dependent children in household | ||||||||
| Yes | 55 (59) | 45.67 (13.63) | 0.910 | 20.90 (10.06) | 0.399 | 9.75 (5.37) | 0.318 | |
| No | 39 (41) | 45.33 (16.77) | 20.09 (9.14) | 8.57 (5.71) | ||||
| Education | ||||||||
| =High school | 34 (36) | 46.03 (14.33) | 0.827 | 18.01 (11.02) | 0.211 | 6.94 (6.22) | 0.835 | |
| High school-college, professional diploma | 32 (34) | 45.88 (15.77) | 21.08 (10.55) | 7.88 (5.81) | ||||
| University and higher | 24 (27) | 45.99 (16.88) | 19.55 (8.59) | 6.94 (5.94) | ||||
| Current working status | ||||||||
| Working full-/part time | 54 (57) | 44.55 (14.65) | 0.586 | 22.40 (10.58) | 0.082 | 9.09 (5.92) | 0.404 | |
| On disability/laid off | 40 (43) | 46.26 (15.22) | 18.94 (8.38) | 8.10 (5.32) | ||||
| Occupation at injury | ||||||||
| Office | 48 (51) | 46.60 (14.66) | 0.878 | 19.75 (8.94) | 0.772 | 9.46 (5.45) | 0.176 | |
| Laborers | 46 (49) | 45.13 (15.27) | 20.31 (9.68) | 7.87 (5.86) | ||||
| Accident involvement due to sleepiness | ||||||||
| Yes | 8 (9) | 45.14 (15.25) | 0.406 | 15.00 (8.40) | 0.090 | 8.00 (5.76) | 0.728 | |
| No | 86 (91) | 49.75 (10.66) | 20.94 (9.47) | 8.73 (5.67) | ||||
| Family difficulties, by DSM-IV-TR | ||||||||
| Yes | 59 (62) | 44.95 (15.07) | 0.626 | 20.59 (8.82) | 0.829 | 9.49 (5.40) | 0.068 | |
| No | 28 (38) | 46.51 (14.84) | 20.15 (10.70) | 7.29 (5.91) | ||||
| Previous WSIB claims | ||||||||
| Yes | 8 (9) | 49.50 (14.83) | 0.435 | 19.25 (10.12) | 0.715 | 9.75 (5.22) | 0.576 | |
| No | 86 (91) | 46.16 (14.97) | 20.54 (9.49) | 8.57 (5.70) | ||||
| Probable/possible malingering, by DSM-IV-TR | ||||||||
| Yes | 14 (16) | 51.64 (13.32) | 0.109 | 15.86 (6.95) | 0.084 | 11.36 (6.16) | 0.060 | |
| No | 74 (84) | 44.72 (14.89) | 20.58 (9.60) | 8.25 (5.48) | ||||
| Tension with employer | ||||||||
| Yes | 34 (36) | 46.62 (13.35) | 0.598 | 19.67 (9.24) | 0.568 | 10.09 (6.07) | 0.067 | |
| No | 60 (64) | 44.92 (15.82) | 20.85 (9.68) | 7.87 (5.31) | ||||
| Tension with insurer | ||||||||
| Yes | 14 (15) | 47.21 (15.33) | 0.650 | 18.29 (10.27) | 0.362 | 9.64 (6.51) | 0.489 | |
| No | 80 (85) | 45.24 (14.76) | 20.81 (9.37) | 8.50 (5.53) | ||||
| Injury-related | Mechanism of injury | |||||||
| Caught, crushed, jumped, pinched | ||||||||
| Yes | 4 (4) | 41.00 (15.66) | 0.667 | 30.50 (12.02) | 0.130 | 4.00 (4.24) | 0.240 | |
| No | 90 (96) | 46.63 (14.99) | 20.28 (9.39) | 8.77 (5.78) | ||||
| Struck by inanimate object | ||||||||
| Yes | 18 (19) | 46.11 (13.63) | 0.856 | 18.77 (6.31) | 0.398 | 9.11 (5.97) | 0.716 | |
| No | 76 (81) | 45.40 (15.30) | 20.84 (10.10) | 8.57 (5.62) | ||||
| Struck by another person | ||||||||
| Yes | 10 (9) | 45.90 (10.33) | 0.935 | 16.30 (7.53) | 0.146 | 9.60 (6.95) | 0.585 | |
| No | 84 (91) | 45.49 (15.43) | 20.93 (9.62) | 8.56 (5.53) | ||||
| Struck against object/structure | ||||||||
| Yes | 16 (17) | 47.75 (14.16) | 0.008 | 19.45 (8.52) | <0.001 | 9.00 (4.14) | 0.864 | |
| No | 78 (83) | 32.37 (17.54) | 30.88 (13.22) | 8.64 (5.80) | ||||
| Exposure to explosion | ||||||||
| Yes | 2 (2) | 41.00 (9.90) | 0.667 | NC** | NC** | 10.00 (5.66) | 0.739 | |
| No | 92 (98) | 45.63 (15.04) | 8.64 (5.69) | |||||
| Motor-vehicle accident | ||||||||
| Yes | 12 (13) | 43.75 (18.32) | 0.660 | 20.25 (8.99) | 0.944 | 8.33 (6.19) | 0.916 | |
| No | 82 (87) | 45.79 (14.48) | 20.46 (9.62) | 8.65 (5.62) | ||||
| Fall from elevation | ||||||||
| Yes | 18 (19) | 41.33 (16.80) | 0.186 | 22.61 (10.92) | 0.280 | 8.72 (6.02) | 0.966 | |
| No | 76 (81) | 46.53 (14.39) | 19.91 (9.12) | 8.66 (5.62) | ||||
| Fall from same level | ||||||||
| Yes | 17 (18) | 51.35 (12.72) | 0.076 | 17.88 (7.99) | 0.223 | 6.94 (4.92) | 0.166 | |
| No | 77 (82) | 44.25 (15.44) | 21.00 (9.75) | 9.05 (5.77 | ||||
| Loss of consciousness | ||||||||
| Yes | 29 (31) | 44.43 (14.67) | 0.352 | 19.56 (11.34) | 0.699 | 9.57 (5.12) | 0.297 | |
| No | 65 (69) | 45.87 (14.22) | 20.22 (9.67) | 8.26 (5.88) | ||||
| Post-traumatic amnesia | ||||||||
| Yes | 21 (25) | 43.62 (15.40) | 0.508 | 21.19 (9.74) | 0.679 | 8.86 (4.70) | 0.865 | |
| No | 73 (75) | 46.08 (14.85) | 20.21 (9.48) | 8.62 (5.94) | ||||
| Previous head trauma | ||||||||
| Yes | 23 (25) | 44.71 (14.52) | 0.174 | 21.50 (8.63) | 0.366 | 9.73 (5.16) | 0.208 | |
| No | 71 (75) | 45.77 (15.66) | 20.19 (9.82) | 8.41 (5.82) | ||||
| Non-specific head MRI or CT findings | ||||||||
| Yes | 27 (32) | 44.04 (14.60) | 0.476 | 20.77 (11.27) | 0.499 | 7.34 (4.87) | 0.188 | |
| No | 57 (68) | 46.55 (15.01) | 19.26 (8.39) | 9.10 (5.76) | ||||
| Not noted^ | ||||||||
| Non-specific (degenerative) neck X-ray/CT findings | ||||||||
| Yes | 47 (58) | 48.15 (15.01) | 0.084 | 19.25 (9.09) | 0.085 | 8.17 (5.67) | 0.689 | |
| No | 35 (42) | 40.60 (12.53) | 24.07 (9.85) | 8.80 (3.71) | ||||
| Not noted^ | ||||||||
| Hematoma/lacerations/head bones’ fracture | ||||||||
| Yes | 22 (23) | 43.36 (14.06) | 0.439 | 22.18 (10.47) | 0.325 | 8.13 (5.35) | 0.616 | |
| No | 72 (77) | 46.19 (15.22) | 19.89 (9.18) | 8.33 (5.78) | ||||
| Comorbid conditions, diagnosed, by self-report | ||||||||
| Arthritis | ||||||||
| Yes | 34 (37) | 46.74 (14.14) | 0.658 | 18.24 (7.90) | 0.125 | 9.71 (5.77) | 0.201 | |
| No | 59 (63) | 45.32 (15.12) | 21.28 (9.71) | 8.14 (5.60) | ||||
| Sleep disorder (any) | ||||||||
| Yes | 10 (11) | 50.50 (13.15) | 0.268 | 19.70 (10.51) | 0.798 | 12.56 (4.67) | 0.033 | |
| No | 84 (89) | 44.94 (15.08) | 20.52 (9.43) | 8.31 (5.64) | ||||
| Diabetes mellitus | ||||||||
| Yes | 5 (5) | 47.00 (20.04) | 0.823 | 20.40 (15.17) | 0.992 | 4.80 (6.38) | 0.117 | |
| No | 89 (95) | 45.45 (14.73) | 20.43 (9.20) | 8.89 (5.58) | ||||
| Heart disease | ||||||||
| Yes | 6 (6) | 44.54 (14.65) | 0.915 | 22.50 (12.45) | 0.584 | 10.33 (6.50) | 0.460 | |
| No | 88 (94) | 45.55 (15.76) | 20.29 (9.33) | 8.56 (5.63) | ||||
| Malignancy | ||||||||
| Yes | 2 (2) | 51.00 (9.90) | 0.603 | 21.50 (10.61) | 0.873 | 9.00 (7.07) | 0.934 | |
| No | 92 (98) | 45.41 (15.03) | 20.41 (9.53) | 8.66 (5.68) | ||||
| DSM-IV-TR disorders | ||||||||
| Adjustment disorder | ||||||||
| Yes | 45 (51) | 47.24 (13.58) | 0.358 | 19.07 (8.95) | 0.442 | 8.62 (6.75) | 0.830 | |
| No | 43 (49) | 44.33 (16.00) | 20.62 (9.82) | 8.88 (7.32) | ||||
| Anxiety disorder | ||||||||
| Yes | 40 (45) | 48.1511.60) | 0.179 | 17.56 (8.70) | 0.042 | 8.55 (5.77) | 0.765 | |
| No | 48 (55) | 43.88 (16.90) | 21.65 (9.55) | 8.92 (5.64) | ||||
| Cognitive disorder | ||||||||
| Yes | 55 (63) | 43.53 (16.10) | 0.060 | 21.27 (10.22) | 0.047 | 8.16 (6.68) | 0.212 | |
| No | 33 (37) | 49.63 (11.68) | 17.27 (7.18) | 9.73 (7.63) | ||||
| Mood disorder | ||||||||
| Yes | 37 (42) | 46.51 (13.32) | 0.709 | 18.59 (6.40) | 0.480 | 8.76 (6.02) | 0.993 | |
| No | 51 (58) | 46.31 (15.90) | 20.23 (10.17) | 8.75 (5.47) | ||||
| Personality traits | ||||||||
| Cluster B | ||||||||
| Yes | 15 (17) | 44.56 (13.88) | 0.300 | 20.25 (8.99) | 0.779 | 8.35 (5.87) | 0.685 | |
| No | 77 (83) | 46.12 (14.32) | 19.78 (10.02) | 7.96 (5.77) | ||||
| Cluster C | ||||||||
| Yes | 42 (47) | 45.98 (14.41) | 0.902 | 21.32 (8.88) | 0.664 | 8.22 (6.88) | 0.055 | |
| No | 50 (53) | 45.58 (15.55) | 20.33 (9.27) | 7.12 (5.33) | ||||
| Somatoform disorder | ||||||||
| Yes | 26 (28) | 46.62 (15.71) | 0.746 | 19.27 (7.85) | 0.724 | 10.35 (6.10) | 0.087 | |
| No | 62 (72) | 45.48 (14.52) | 20.05 (9.98) | 8.08 (5.39) | ||||
| Sleep disorder | ||||||||
| Yes | 7 (10) | 44.86 (15.53) | 0.859 | 21.57 (11.90) | 0.608 | 11.40 (5.52) | 0.107 | |
| No | 81 (90) | 45.90 (14.83) | 19.66 (9.17) | 8.35 (5.62) | ||||
| Substance-related disorder | ||||||||
| Yes | 13 (15) | 40.92 (14.28) | 0.198 | 22.42 (9.50) | 0.302 | 7.69 (4.48) | 0.469 | |
| No | 75 (85) | 46.66 (14.82) | 19.40 (9.33) | 8.93 (7.61) | ||||
| Symptom load | ||||||||
| Balance issues | ||||||||
| Yes | 44 (47) | 47.16 (14.59) | 0.324 | 18.00 (7.99) | 0.021 | 7.98 (5.34) | 0.268 | |
| No | 50 (53) | 44.10 (15.22) | 22.52 (10.23) | 9.28 (5.92) | ||||
| Bodily pain | ||||||||
| Yes | 32 (34) | 46.22 (14.61) | 0.750 | 18.72 (10.05) | 0.210 | 7.56 (5.67) | 0.174 | |
| No | 62 (66) | 45.18 (15.19) | 21.33 (9.15) | 9.24 (5.62) | ||||
| Cognitive complaints | ||||||||
| Yes | 67 (71) | 47.81 (14.40) | 0.019 | 19.46 (8.18) | 0.115 | 9.11 (5.70) | 0.244 | |
| No | 27 (29) | 39.89 (14.98) | 22.92 (12.08) | 7.59 (5.53) | ||||
| Mood disturbance | ||||||||
| Yes | 62 (66) | 45.71 (14.43) | 0.873 | 19.89 (17.67) | 0.448 | 8.32 (5.77) | 0.410 | |
| No | 32 (34) | 45.19 (16.08) | 21.47 (10.99) | 9.34 (5.49) | ||||
| Head and/or neck pain | ||||||||
| Yes | 87 (93) | 45.84 (14.63) | 0.485 | 19.88 (9.37) | 0.051 | 8.52 (7.30) | 0.359 | |
| No | 7 (7) | 41.71 (19.20) | 27.14 (8.95) | 10.57 (5.03) | ||||
| Photo-/phonophobia | ||||||||
| Yes | 14 (15) | 49.86 (13.05) | 0.242 | 19.29 (8.58) | 0.627 | 9.07 (5.90) | 0.776 | |
| No | 80 (85) | 44.78 (15.18) | 20.63 (9.68) | 8.60 (5.66) | ||||
| Sleep-related issues | ||||||||
| Yes | 59 (63) | 44.25 (14.50) | 0.284 | 20.44 (9.93) | 0.981 | 7.36 (5.98) | 0.003 | |
| No | 35 (37) | 47.69 (14.77) | 20.40 (8.86) | 10.89 (5.71) | ||||
| Snoring (STOP-Bang) | ||||||||
| Yes | 71 (76) | 45.03 (14.47) | 0.890 | 20.61 (9.57) | 0.503 | 8.46 (5.77) | 0.859 | |
| No | 23 (24) | 44.50 (16.74 | 19.05 (7.52) | 9.10 (2.24) | ||||
| Observed pause in breathing in sleep (STOP-Bang) | ||||||||
| Yes | 18 (19) | 51.17 (10.25) | 0.046 | 18.83 (8.65) | 0.460 | 10.94 (5.76) | 0.086 | |
| No | 76 (81) | 43.37 (15.51) | 20.23 (9.27) | 8.40 (5.52) | ||||
| Medication intake | ||||||||
| Antihistamines | ||||||||
| Yes | 23 (26) | 48.48 (14.14) | 0.278 | 19.78 (10.76) | 0.708 | 7.78 (5.49) | 0.390 | |
| No | 71 (74) | 44.58 (15.15) | 20.64 (9.11) | 8.96 (5.73) | ||||
| Benzodiazepines | ||||||||
| Yes | 12 (13) | 48.00 (13.99) | 0.543 | 16.25 (9.35) | 0.102 | 7.33 (5.58) | 0.384 | |
| No | 82 (87) | 45.17 (15.11 | 21.05 (9.41) | 8.87 (5.68) | ||||
| Narcotic analgetics | ||||||||
| Yes | 21 (22) | 47.19 (13.80) | 0.566 | 18.57 (8.80) | 0.311 | 9.33 (6.67) | 0.546 | |
| No | 73 (78) | 45.05 (15.29) | 20.97 (9.68) | 8.48 (5.38) | ||||
| Tricyclic antidepressants | ||||||||
| Yes | 27 (29) | 51.74 (12.30) | 0.009 | 18.96 (7.64) | 0.342 | 9.15 (6.49) | 0.606 | |
| No | 67 (71) | 43.02 (15.24) | 21.03 (10.15) | 8.48 (5.34) | ||||
| Serotonin reuptake inhibitors (SSRI) | ||||||||
| Yes | 14 (15) | 44.43 (15.03) | 0.766 | 18.57 (13.05) | 0.430 | 9.29 (4.80) | 0.662 | |
| No | 80 (85) | 45.73 (14.99) | 20.76 (9.50) | 8.56 (5.82) | ||||
| Recreational substance use | ||||||||
| Yes | 9 (12) | 43.56 (10.61) | 0.625 | 21.00 (7.53) | 0.853 | 9.11 (5.18) | 0.869 | |
| No | 83 (88) | 46.08 (15.03) | 20.38 (9.68) | 8.78 (5.71) | ||||
| Sleep timing, bed time (weekday) | ||||||||
| Regular (<60 min) | 50 (53) | 44.37 (14.77) | 0.434 | 22.36 (9.10) | 0.278 | 8.40 (5.52) | 0.356 | |
| Irregular | ||||||||
| 60-120 min | 35 (37) | 52.54 (16.44) | 19.27 (8.80) | 11.40 (5.62) | ||||
| >120 min | 9 (10) | 48.69 (15.88) | 20.32 (10.78) | 9.87 (6.67) | ||||
| Wake timing (weekday) | ||||||||
| Regular (<60 min) | 63 (67) | 49.44 (15.88) | 0.193 | 21.21 (9.10) | 0.129 | 9.78 (5.88) | 0.246 | |
| Irregular | ||||||||
| 60-120 min | 29 (31) | 46.58 (15.42) | 19.41 (8.19) | 9.40 (6.49) | ||||
| >120 min | 2 (2) | 53.44 (12.77) | 18.55 (11.77) | 10.87 (5.67) | ||||
| Taking nap during the day | ||||||||
| Yes | 59 (64) | 46.08 (15.34) | 0.637 | 19.73 (9.39) | 0.350 | 10.02 (8.56) | 0.002 | |
| No | 34 (36) | 44.56 (14.34) | 21.65 (9.70) | 6.29 (4.91) | ||||
| Completion of assessment in the afternoon vs morning or night | ||||||||
| Yes | 41 (44) | 41.54 (14.69) | 0.027 | 21.59 (11.07) | 0.149 | 8.81 (5.16) | 0.853 | |
| No | 53 (56) | 48.34 (14.48) | 18.79 (7.42) | 8.58 (6.09) | ||||
Table 1a: Characteristics of the study population and corresponding fatigue, alertness and daytime sleepiness scores for binary and categorical variables.
| Variables | Mean (SD)/median (Q3-Q1) | FSS score mean (SD) Rho | P-value | THAT score mean (SD) Rho | P-value | ESS score mean (SD) Rho | P-value |
|---|---|---|---|---|---|---|---|
| Age, years | 45.2 (9.94) | 0.103 | 0.301 | -0.21 | 0.845 | -0.003 | 0.974 |
| Weekly income, $CAD | 1056 (510) | -0.206 | 0.047 | 0.089 | 0.392 | -0.045 | 0.665 |
| Time since injury, days | 197 (416-139) | -0.243 | 0.021 | 0.114 | 0.275 | -0.156 | 0.133 |
| Number of comorbid conditions | 2.22 (1.04) | 0.16 | 0.123 | -0.106 | 0.314 | 0.087 | 0.402 |
| Patient-reported (PR) states | |||||||
| Insomnia (ISI) | 17.47 (6.32) | 0.52 | <0.001 | -0.394 | <0.001 | 0.047 | 0.652 |
| Anxiety (HADS-A) | 10.71 (4.74) | 0.327 | 0.001 | -0.376 | <0.001 | 0.127 | 0.222 |
| Depression (PHQ-9)* | 14.31 (6.31) | 0.551 | <0.001 | -0.454 | <0.001 | 0.123 | 0.239 |
| Pain (VAS-P), current | 5.02 (2.40) | 0.263 | 0.011 | -0.257 | 0.013 | -0.066 | 0.529 |
| Restless legs (RLQ) | 3.15 (2.42) | 0.219 | 0.035 | -0.339 | <0.001 | 0.064 | 0.541 |
| Narcolepsy (SNS) | 3.15 (2.42) | 0.051 | 0.626 | -0.092 | 0.378 | -0.066 | 0.526 |
| Total number of sleep-related breathing disorder risk factors (STOP-Bang) | 4.19 (1.67) | -0.013 | 0.898 | 0.033 | 0.757 | 0.063 | 0.540 |
| Substances | |||||||
| Alcohol intake, daily (portion(s) of beer, wine, or liquor) | 0.40 (1.08) | -0.025 | 0.813 | -0.025 | 0.813 | 0.122 | 0.242 |
| Coffee, servings a day | 2.10 (1.65) | 0.066 | 0.528 | -0.075 | 0.474 | 0.048 | 0.675 |
| Number of prescribed medications | 1.10 (0.96) | 0.189 | 0.069 | -0.156 | 0.136 | 0.025 | 0.807 |
| Body mass index | 28.68 (5.14) | -0.084 | 0.420 | 0.157 | 0.133 | -0.051 | 0.624 |
| Total sleep time | 6.22 (2.16) | -0.066 | 0.528 | -0.136 | 0.197 | 0.010 | 0.931 |
| Sleep efficiency | 0.71 (0.21) | -0.117 | 0.210 | 0.14 | 0.181 | -0.155 | 0.138 |
Table1b: Characteristics of the study population and corresponding fatigue, alertness and daytime sleepiness scores for continuous variables.
▪ Bivariate analyses
The mean total FSS score was 45.53 ± 14.92, with 43.02 ± 15.89 for men and 48.81 ± 12.77 for women (where scores ≥ 36 indicate clinically significant fatigue [25]). The mean alertness score on THAT was 20.43 ± 9.49, with 21.30 ± 9.22 for men and 19.06 ± 8.88 for women (where scores ≤ 20.5 indicate impaired alertness [26]). The mean sleepiness score on the ESS was 8.67 ± 5.66, with 8.53 ± 5.69 for men and 8.89 ± 5.70 for women (where scores ≥10 indicate excessive sleepiness [27]). The total FSS scores were negatively associated with the total THAT scores (p<.001) and positively with the total ESS scores (p=.014). There were no associations between the total THAT and total ESS scores (p=.257). The internal consistency of the FSS in our sample, as measured by Cronbach’s α, was .95, the THAT was .85, and the ESS was .78.
▪ Fatigue
Persons injured by striking against an object/ structure had significantly higher FSS total scores (47.75 ± 14.16 vs 32.37 ± 17.51, p=.008) than those with other injury mechanisms. Participants with cognitive complaints had significantly higher FSS scores (47.81 ± 14.40 vs 39.89 ± 14.98, p=.019) than those without. There were significant differences in the FSS scores between persons who did and did not take tricyclic antidepressants (TCAs) and those who did and did not paused during breathing in sleep (51.74 ± 12.30 vs 43.02 ± 15.24, p=.009 and 51.17 ± 10.25 vs 43.37 ± 15.51, p=.046, respectively). Participants who completed PR measures in the afternoon had significantly lower scores (41.54 ± 14.69 vs 48.34 ± 14.48, p=.027) than those who completed them in the morning or night. The Spearman’s correlation coefficients for continuous variables were as follows: the FSS scores were negatively correlated with TSI (rho=-.243, p=.021) and pre-injury weekly salary (rho=-.206, p=.047), and positively with number of injuries within the past five years (rho=.317, p=.001), insomnia (rho=.520, p<.001), depression (rho=.551, p<.001), anxiety (rho=.327, p=.001), pain (rho=.263, p=.011) and restless legs (RL) (rho=.219, p=.035). There were no significant effects among the other independent variables.
▪ Alertness
Participants injured by striking against an object/ structure had significantly lower THAT total scores (19.45 ± 8.52 vs 30.88 ± 13.22, p<.001) than those with other injury mechanisms. THAT scores differed significantly between persons who did and did not report balance issues and head and neck pain at the time of assessment (18.00 ± 7.99 vs 22.52 ± 10.23, p=.021 and 19.88 ± 9.37 vs 27.14 ± 8.95, p=.051, respectively). Persons with DSM-IV-TR anxiety disorder had significantly lower THAT scores (17.56 ± 18.70 vs 21.65 ± 9.55, p=.042), and those with cognitive disorders significantly higher ones (21.27 ± 10.22 vs 17.27 ± 7.18, p=.047), than those without these diagnoses. The Spearman’s correlation coefficients for continuous variables showed the THAT scores were negatively associated with insomnia, depression, anxiety (rho=.- 394, rho=.-454 rho=.-376, respectively, p<.001), pain (rho=.-257, p=.013) and RL (rho=.-339, p<.001). There were no significant effects among the other independent variables.
▪ Sleepiness
Persons born in Canada and those with English as first language had significantly higher ESS total scores (9.79 ± 5.48 vs 4.26 ± 4.05, p<.001 and 9.35 ± 5.54 vs 5.59 ± 5.50, p=.012, respectively) than others. Persons who reported being diagnosed with sleep disorder had significantly higher ESS scores (12.56 ± 4.67 vs 8.31 ± 5.64, p=.033) than those without these diagnoses. The ESS scores differed significantly between persons who reported sleep difficulties and those who did not (7.36 ± 5.98 vs 10.89 ± 5.71, p=.003) and those napping during the day and those who did not (10.02 ± 8.56 vs 6.29 ± 4.91, p=.002). No other significant correlations were observed.
The complete results of ANOVA for binary and categorical variables and the Spearman’s correlation coefficients for continuous variables and their associated values with fatigue, alertness and sleepiness are given in Table 1a,b.
▪Multivariable regression analyses
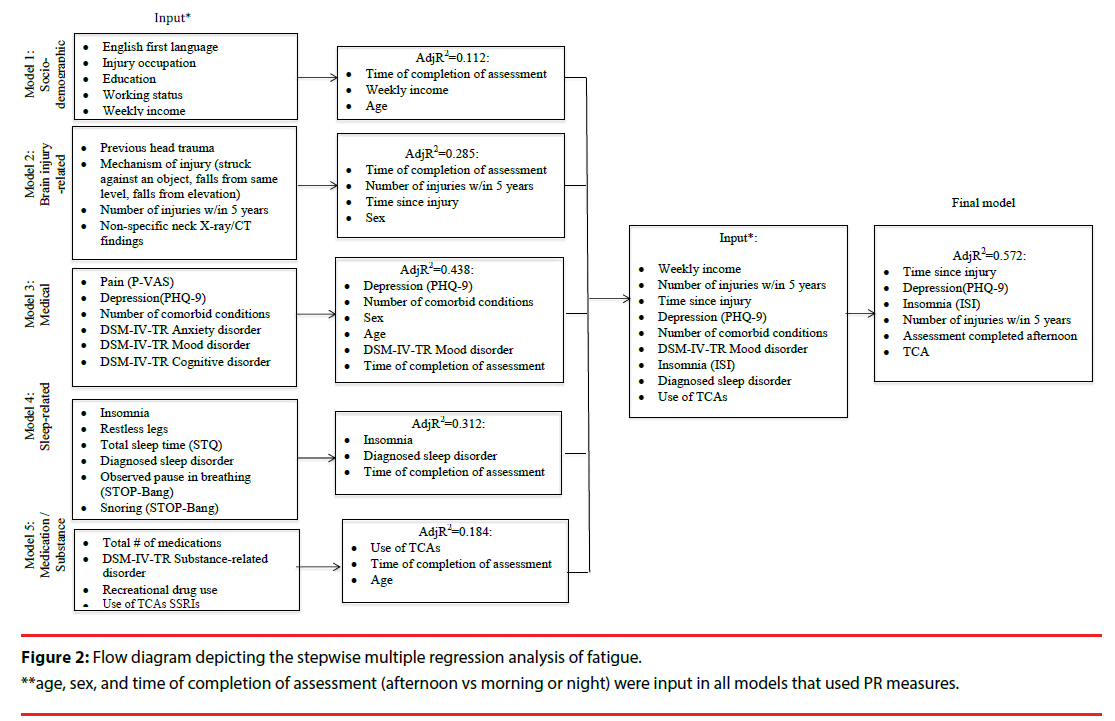
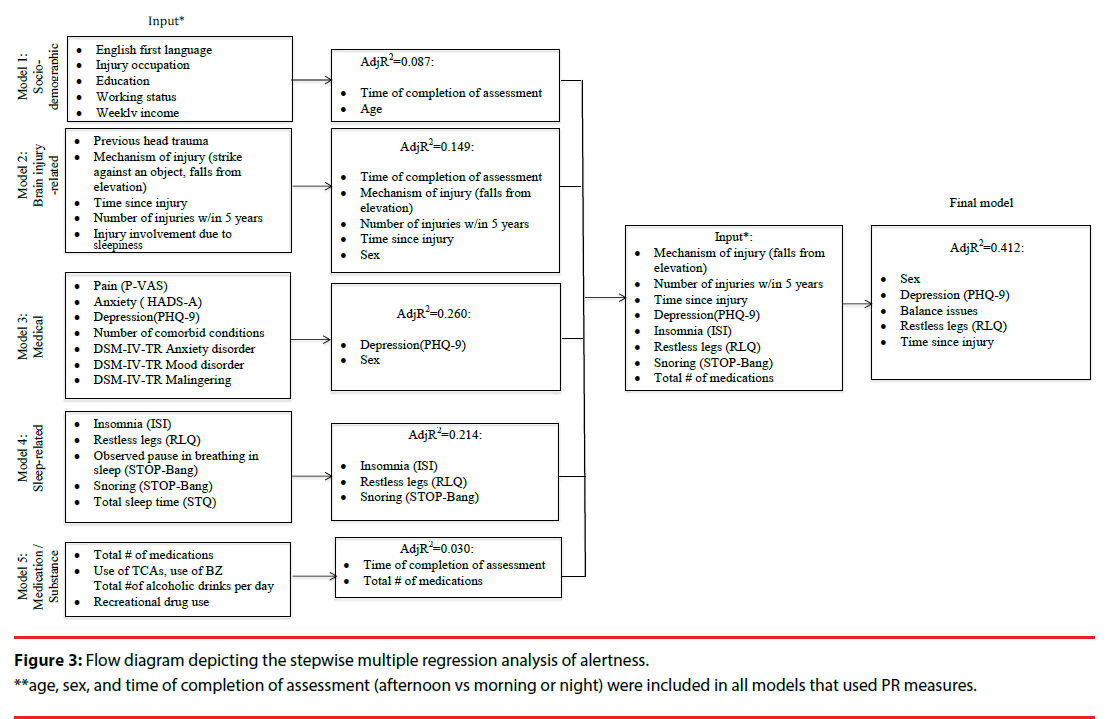
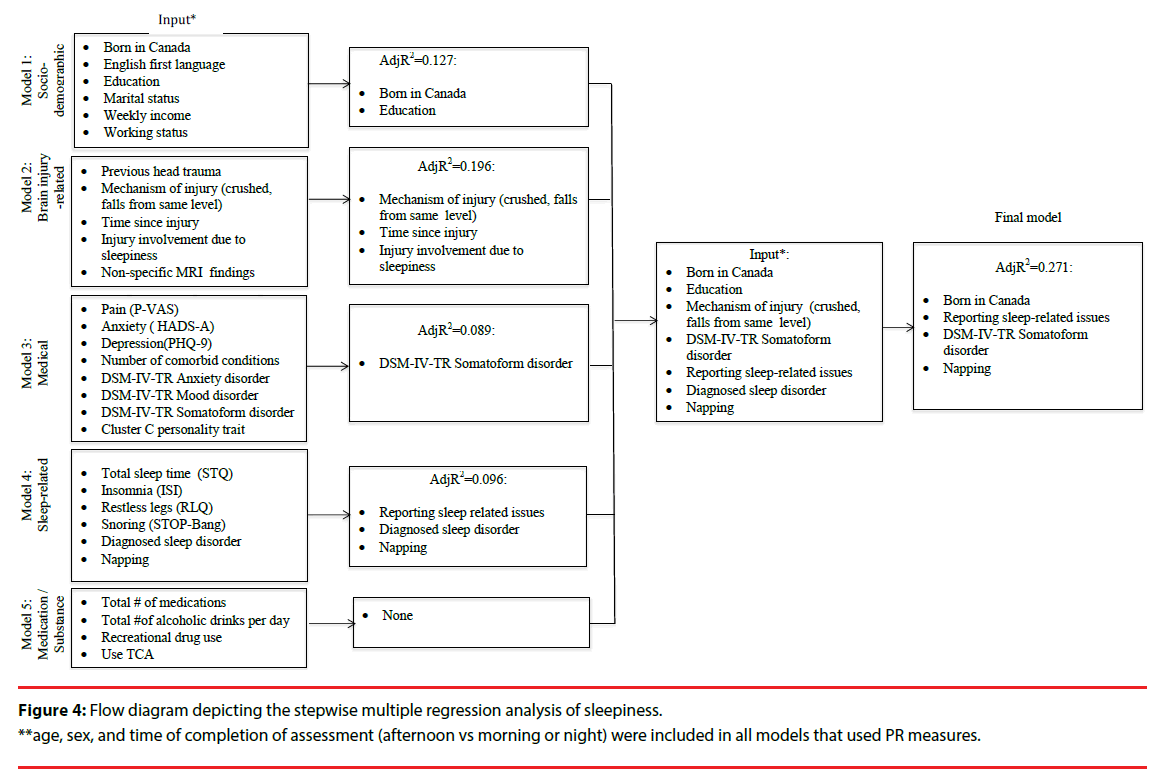
Several multivariate linear regression analyses were used to assess how the covariates within sociodemographic, brain-injury-, medical, sleep-, and medication/substance effect-related categories were associated with the outcome measures (i.e. fatigue, sleepiness, and alertness). We fitted our stepwise regression models on the basis of the bivariate analyses, associations reported in the literature, and pre-defined hypotheses (Figures 2-4). All our models were adjusted for age and sex, and those that utilized the PR measure were also adjusted for assessment time. We did not observe VIF > 4 for any covariate, suggesting that collinearity did not contribute to the change in regression estimates. The final regression model tested covariates of fatigue, alertness, and sleepiness identified in the five preliminary models at p ≤ .1. Following stepwise selection and using a threshold level of significance of p ≤ .05, the final fully-adjusted model of fatigue explained 57.2% of the variance and contained six variables: depression severity (β=.947, p<.001), TSI (β=- .004, p<.001), insomnia (β=.529, p=.006), completion of the PR measure in the afternoon (vs morning or night) (β=-7.083, p=.008), use of TCAs (β=6.232, p=.015) and number of injuries within the past five years (β=7.443, p=.010) (Table 2). The final fully- adjusted model of alertness explained 41.2% of the variance and contained four variables: depression severity (β=- 0.694, p<.001), TSI (β=0.002, p=.044), balance issues (β=-5.816, p=.009), RL severity (β=-2.002, p=.003) and male sex (β=3.439, p=.031). Finally, the final fully- adjusted model of sleepiness explained 27.1% of the variance and contained four variables: born in Canada (β=3.750, p<.001), napping during the day (β=2.893, p=.009), reporting sleep-related issues (β=-3.311, p=.009), and DSM-IV-TR somatoform disorder (β=2.423, p=.037) (Table 2, Appendix C).
| Fatigue model | Variable | β Coefficient | SE | P Value | Partial R2 | Model R2/AdjR2 |
| #1 Socio-demographic | Age | 0.0006 | 0.0003 | 0.071 | 0.016* | 0.016 |
| #2 Clinical | Depression | 0.947 | 0.205 | <0.001 | 0.356 | 0.372 |
| Use of tricyclic antidepressants | 6.232 | 2.412 | 0.015 | 0.042 | 0.414 | |
| #3Injury- related | Time since injury | -0.004 | 0.001 | <0.001 | 0.083 | 0.497 |
| #4 Psychosocial | # injuries w/in 5 yr | 7.443 | 2.393 | 0.010 | 0.033 | 0.530 |
| #5 Sleep-/circadian rhythm-related | Insomnia | 0.529 | 0.230 | 0.006 | 0.047 | 0.577 |
| Afternoon assessment (vs morning or night) | -7.083 | 2.105 | 0.008 | 0.041 | 0.600/ 0.572 |
|
| Alertness model | Variable | β Coefficient | SE | P Value | Partial R2 | Model R2/AdjR2 |
| #1 Socio-demographic | Sex, male | 3.439 | 1.566 | 0.031 | 0.031 | 0.031 |
| #2 Clinical | Depression | -0.694 | 0.120 | <0.001 | 0.255 | 0.286 |
| Balance issues | -5.816 | 1.591 | 0.009 | 0.052 | 0.338 | |
| #3 Injury- related | Time since injury | 0.00165 | 0.0008 | 0.044 | 0.037 | 0.375 |
| Falls from same level | 3.419 | 2.112 | 0.109 | 0.017* | 0.375 | |
| #4 Sleep-/circadian rhythm-related | Restless legs | -2.002 | 0.626 | 0.003 | 0.063 | 0.438/ 0.412 |
| Daytime sleepiness model | Variable | β Coefficient | SE | P Value | Partial R2 | Model R2/ AdjR2 |
| #1 Socio-demographic | Born in Canada | 3.750 | 1.361 | <0.001 | 0.135 | 0.135 |
| #2 Clinical | Diagnosed sleep disorder | 2.985 | 1.718 | 0.086 | 0.025* | 0.160 |
| #3 Psychosocial | Axis-IV-TR somatoform disorder | 2.423 | 1.145 | 0.037 | 0.038 | 0.198 |
| #4 Sleep-/circadian rhythm-related | Reported sleep issues | -3.311 | 1.114 | 0.009 | 0.064 | 0.262 |
| Napping | 2.893 | 1.118 | 0.009 | 0.068 | 0.305/ 0.271 |
|
| *p>.05, not includedin R2 | ||||||
Table2. Summary of the stepwise multiple regression analysis for the final models of fatigue, alertness and daytime sleepiness
Discussion
Fatigue, impaired alertness and daytime sleepiness are increasingly pervasive and clinically relevant manifestations in patients with concussion/mTBI [32-34]. Discerning patterns of integrative relationships among elements is critical for defining complex states better, and proper differentiation between them is essential for future progress in developing interventions. Grouping elements across simpler organizational themes allowed us to begin with associations, followed by statistical models at various hierarchical levels of aggregation, to explicate the physiological, pathological and behavioral processes of fatigue, alertness, and daytime sleepiness in concussion/mTBI. The study showed that although these three states are different constructs in concussion/mTBI, they share covariates. Fitting the final statistical models with variables spanning across individual models at the p ≤ .1 aggregation level (i.e., to provide a more generic discussion) revealed the elements across levels of organization, which were initially viewed as associations, but also suggested temporal mechanisms to explicate the basis of the three states.
Our final model showed that fatigue can be explained by depressive illness and/or its treatments, insomnia disorder, the influence of the circadian and homeostatic drive at the time of reporting, and environmental state, potentially depicting protection from harm at work. Likewise, alertness shared the depressive illness aggregate, and its explanatory factors spanned across components related to brain health (i.e., restless leg’s, balance issues), together explaining 41% of the variance. Conversely, daytime sleepiness, conceptualized as “representing different levels of ‘somnificity’ that most people encounter as part of their daily lives” and defined as “the general characteristic of a posture, activity and situation that reflects its capacity to facilitate sleeponset in the majority of subjects” [35], is associated with the cultural/environmental component, certain behaviours, sleep-related variables, and somatization in general.
▪ Fatigue
Multiple factors of fatigue (i.e., depression, anxiety, insomnia, etc.) are consistently reported in populations with various neurological disorders [36,37]. The extent to which these factors are etiologically related in patients with concussion/mTBI remains unclear. Our depression measure (PHQ-9) features a sleep item (i.e., trouble falling or staying asleep, or sleeping too much) within it, which, when it was removed from the analyses, resulted in a stronger covariate effect of insomnia on both, fatigue and alertness, and a weaker effect of depression. The high coincidence and overlapping depressive and insomnia symptoms may suggest a common neurobiology, where mechanisms contributing to abnormal sleep patterns in concussion/ mTBI making the individual more susceptible to depression [18]. Likewise, the TCA covariate effect is difficult to interpret: TCAs differ not only in their relative effects in blocking serotonin versus norepinephrine reuptake and the degree of antagonism of H1 histamine receptors, but also in their effects on depressed patients, which are reported to be variable [38,39]. Nevertheless, our results are important, as earlier research reported that this group of medications can activate the cytokine system, as manifested by sickness, depressive behavior, and chronic fatigue [40]. The immunomodulatory effects [41] of psychoactive medications as it relates to fatigue should be investigated further in concussion/mTBI.
We found that fatigue is less profound during the day than the morning or night, consistent with the energy allocation model, outlining fluctuations in performance capacity over the day [42], Earlier research suggested that a more regular a more regular day-to-day pattern of rest and activity relates to lower fatigue and depression scores [43-45]. Variation in day-to-day rest and activity pattern in persons with concussion/mTBI is, therefore, important to consider in future studies investigating perceived states.
The covariate effect of TSI on fatigue is consistent with previous research [46,47], reinforcing the concept of time in concussion-related fatigue. Our findings regarding associations between fatigue and number of work-related injuries within the past five years in the final regression model adjusted for age, sex, weekly income, depression, insomnia, number of comorbid conditions, medication effect, and TSI, need to be replicated in a longitudinal study before causal conclusions can be drawn; they could imply, however, that fatigued persons are more at risk of unsafe performance and inappropriate strategies in the workplace and therefore, more at risk of injury [48,49]. Given that almost every second worker performed shift work at the time of their injury, and almost 8% believed their involvement in the most recent accident with brain injury outcome was due to sleepiness, such implications are likely, and suggest that frequent injuries indicate the state of the individuals’ vigilance networks [50]. Etiologically, however, such factors as repetitive injuries could decrease a person’s responsiveness to stressors associated with a new injury; subsequent perpetuating factors could catalyze progression to chronic fatigue and generate sickness behavior [51]. These findings support the compelling evidence that fatigue may compromise safety [51] and suggest its covariates need to be managed carefully in concussion/mTBI.
▪ Alertness
The covariate effect of depression and TSI on fatigue and alertness observed in our results supports the notion of shared variance between these constructs in concussion/mTBI. Absolute value correlations between fatigue and alertness in our sample were moderate (rho=-.61); equivalent, in variance terms, to a shared variance of 10-50% [52]. Previous studies raised several hypotheses including neurotransmitter imbalance, dysregulation of the hypothalamic- pituitary-adrenal axis, and genetic polymorphism to explain the mechanisms involved in post- concussive sleep regulation and psychiatric illness [53]. Thus, recurrence or worsening of sleep as a result of concussion/mTBI could account for the relapse of mood symptoms and also treatment resistance [54]. Since around 69% of our sample expressed clinical insomnia, continued study of sleep in concussion/mTBI is appropriate.
Balance issues and RL (a sensory-motor disorder, a central characteristic of which is worsening of symptoms during the evening or night [54]) were independently associated with decreased alertness. Generally, imbalance results from disorders of the spinal cord (spinocerebellar pathway) or vestibular input, the integration of these inputs in the brainstem, or the motor output to the spinal neurons controlling axial or proximal muscles [55]. Diffuse axonal injury (DAI) as a result of strain in the axonal direction during the injury event with mechanism such as struck against an object or structure [56], can affect any combination of central vestibular pathways in the brain stem, cortico-cerebral or cerebellar tracts, resulting in symptoms of imbalance [57], reinforcing our findings and accounting for our observed relationship. Restless legs symptoms associate with core body temperature, and salivary melatonin secretion in the general population [58]. While we did not collect information about core body temperature or salivary melatonin secretion, the strong indication of circadian rhythm disturbances in our sample [18], could reasonably explain the results.
Alertness appeared to be sex-dependent in our final fully adjusted model, with males being more alert than females. In the general population, males are reportedly more vigilant than females throughout life [59-61]. However, other researchers observed that differences in alertness between males and females vary diurnally [62], and are result of the less intense endogenous control of circadian rhythms in women, with environmental and sociocultural influences having a modulating role [62]. Our results reinforce the notion that research focusing on unobservable constructs that are subject to homeostatic or chronophysiological functions should control for time of data collection and sex. At the same time, sex differences were observed in our final model of alertness, controlled for age, time of assessment, and other relevant covariates. Further research focusing on differences between men and women in homeostatic sleep drive, as an opposed function to circadian-dependent drive for alertness during waking day, is timely.
▪ Sleepiness
It is generally accepted that degree of daytime sleepiness is directly related to the duration of nocturnal sleep and wakefulness, a primary factor of homeostatic sleep drive [63]. Individual differences in tolerance of sleep loss are well known [64,65]. We did not collect data on duration of wakefulness (i.e., sleep-wake history) the day preceding investigation, to understand the strength of homeostatic sleep drive or perceived level of sleepiness in our research participants. This might explain why the majority of the variance in daytime sleepiness remained unexplained in our fully adjusted multivariable model, and may (if prolonged) be responsible for the elevated mean ESS score for the entire cohort of patients in this study (both males and females), comparable to that reported for the general community (8.67 ± 5.66, with 8.53 ± 5.69 for men and 8.89 ± 5.70 for women; vs 6.3 ± 3.5 and - 5.9 ± 2.2, respectively [66,67]. In TBI patients, central nervous system pathology with hypocretin/orexin deficiency is thought to underlie daytime sleepiness [68]. We did not take cerebrospinal fluid samples to examine this relationship; however, we had no narcolepsy cases in our sample, as determined by the Swiss Narcolepsy Scale (SNS) and clinical examination. In all cases of excessive sleepiness, our participants also had elevated scores on the STOP-Bang measure, which could explain the elevated sleepiness. The mean ESS scores in our sample were similar to those reported in larger population-based studies in patients with respiratory disturbance index (RDI) of 15- 30 [69]. Interestingly, higher rates of daytime sleepiness are reported in selected populations: when the ESS was completed by 740 dayworkers in eight industrial plants in Israel, 23% of respondents had scores greater than ten [70]. Hesselbacher and colleagues reported that the ESS scores are influenced not only by RDI, but also by sex, ethnicity, and body morphometry [71]. Being born in Canada and having English as first language were highlighted in our study as covariates of daytime sleepiness. While they cannot be regarded as substitutes for ethnicity, they may suggest a cultural mechanism (i.e., different subgroups of respondents interpret items differently, or social situations “as a passenger” or “in a theatre or a meeting” refer to specific social contexts, the frequency of which might not be sufficient to allow some cultural subgroups in Canada to make a valid assessment) [72]. Furthermore, sleepy persons for whom English is not a first language, or who do not read often, might choose “would never doze” for that question, answering it directly or hypothetically. The cultural dimorphism revealed in our study with regard to daytime sleepiness and its measurement, may therefore challenge the universality of the daytime sleepiness construct depicted by the ESS across non-clinical and clinical populations with various cultural backgrounds and should therefore be further explored. This is particularly relevant in a Canadian context, given the linguistic diversity of this nation and the recent increase in the number of immigrants with languages other than English [73-75].
Napping in our study of middle-aged persons with concussion/mTBI has been identified as a covariate of daytime sleepiness, independent of age, sex, education, sleep disorder, somatoform disorder or mechanism of brain injury. Should napping be viewed as potentially protective or as an independent covariate of risk for daytime sleepiness? Given our cross-sectional study design, we cannot answer this question directly. Our complex association matrix showed no association between daytime sleepiness and either multimorbidity or multipharmacy, mechanism of injury, LOC, PTA, RL severity, or sex, but moderate associations with reported sleep-related issues at assessment. Therefore, our evidence continues to support the notion that napping in the chronic phase of concussion does not improve daytime sleepiness, but could adversely affect the ability to fall asleep and could be associated with less slow-wave sleep, which is viewed as most restorative [76,77]. Future longitudinal studies are needed to validate our results.
Finally, somatoform disorder, in which patients present with multiple physical complaints to different organ systems [78], remained in our final model as an independent covariate of daytime sleepiness. Patients with multiple somatic complaints that cannot be explained by a known medical condition or by the effect of alcohol/recreational or prescription drugs constitute 5% of the general population [79], but in our sample such disorders were documented in the medical files of 28%, which is significantly higher. It cannot be determined whether this high prevalence is attributable to the diffuse brain injury, comorbid disorders, drug interactions due to regimes initiated independently by different treatment providers, or the impulsiveness of patients with somatization disorder in demanding formal diagnosis after concussion/mTBI. Our methods can only undermine the hypothesis rather than answer this question. Longitudinal studies with baseline assessment as early as possible after the injury are needed.
Strength and limitations
Strength of this study is its interdisciplinary approach, crossing neural, psychosocial, clinical, and behavioral levels of complex constructs that could be context-dependent, making it possible to identify associations on multiple levels and thus improving the quality of inductive inferences. A strength of the correlative approach lies in identifying novel previously-undescribed associations that could be replicated and worthy of further study. The multivariate techniques used allowed the constructs to be reduced to more meaningful functional parts. During the development phase of the project we formulated concepts and hypotheses based on a synthesis of relevant discoveries across various disciplines [16,80]. Abstract constructs arising from this research provide a means of understanding highly complex perceived states, helping us to think about elements from different levels of inference and thus increasing the comprehensiveness and relevance of hypotheses concerning complex unobservable constructs. Also, the diagnoses of concussion/mTBI were made by a team of clinicians trained in neurology, psychiatry, psychology and other relevant disciplines. All diagnoses were provided to the insurer and therefore indicate liability.
Recruitment to the study was not random; the selected participants had been gainfully employed at the time of concussion/mTBI and all had job to return to, but continued to experience symptoms interfering with their functioning. In addition to the collected data pertinent to our research questions, we had access to all medical assessments and pre-morbid histories. While we had reasonably high response rates, many potential research participants were not enrolled owing to their lack of informed consent or interest in the research. We retrospectively reviewed the charts of the consecutive list of participants assessed in the same clinic in 2003 to ensure our sample was representative. Nevertheless, there could have been selection bias towards those with more significant distress, those who experienced less significant physical or cognitive limitations, or those who wanted to understand the cause of their ongoing difficulties; although bias could have lain in the other direction. Nevertheless, the TSI, injury severity, socio- demographic and occupational characteristics of our study group suggest that our findings are likely to be generalizable to an entire recruitment cohort of insured persons with persistent symptoms after concussion/mTBI.
We used PR measures to study fatigue, alertness, and sleepiness in concussion/mTBI. Earlier study indicates it is easier to perceive fatigue or lack of energy than any degree of sleepiness [81]. The perception of sleepiness can also be influenced by the duration of sleep deprivation (i.e., individuals could have adjusted to their long-term impairment, making them less likely to recognize their degree of sleepiness, instead reporting tiredness). To mitigate these issues, we attempted to distinguish covariates of daytime sleepiness, fatigue, and impaired alertness by collecting a variety of data related to history of sleep function, and we also utilized standardized scales with items enabling us to differentiate among the three constructs. Nevertheless, it remains unclear how well the items within our measures reflected the constructs under study as we relied on how the measures of complex constructs were depicted by those who developed them [25-27]. We provided information on the internal consistency of all PR data, and the results show their use in our sample to have been appropriate. Non-response bias occurred at 5% on average across all measures, which is low for PR measurements. Nonetheless, further description of the psychometric properties of the FSS, THAT and ESS in concussion/mTBI is needed, especially those pertaining to construct validity and reliability. Although our sample size was adequate to allow accurate estimation of regression coefficients, standard errors, and confidence intervals in all linear regression models, and we had reasonably high response rates, many potential research participants were not enrolled owing to their lack of informed consent or interest in the research. Confirmation of our results in larger studies is warranted. Finally, this study highlighted the factors that were associated with perceived states at the moment of investigation (i.e., cross-sectional relationship); the longitudinal relationships between these factors and studied outcomes remain to be determined.
Conclusion
Our study suggests that fatigue, alertness and daytime sleepiness in concussion/mTBI may not be solely explained by socio-demographic, brain-injury-, medical-, sleep-related variables, or medication/drug effects. The analyses across domains provide unique opportunities for future research investigating complex perceived states that tend to be multiply determined. Similar conclusions were made earlier in Project Leonardo [82], an interdisciplinary disease and care management model developed for patients with heart failure and diabetes, pointing to the value of multidisciplinary collaborations and evaluations of all aspects of patient health and functioning when faced with persistent symptoms in chronic disorders. Sufficient understanding of how fatigue, alertness, and daytime sleepiness are related in concussion/mTBI is important for a broader health and safety management system, in particular regarding release to return to work after injury. Further attention to potential pathways in longitudinal studies is key to determining how to manage these states and also to the search for effective solutions.
Conflict of Interest and Source of Funding
The authors have no conflict of interest to declare pertaining to this work.
Our study had no external funding source. The first author was supported by the 2013/2015 Frederick Banting and Charles Best Doctoral Research Award from the Canadian Institutes of Health Research and the postdoctoral fellowship from the Faculty of Occupational Science and Occupational Therapy at the University of Toronto. Angela Colantonio was supported by the Canadian Institutes for Health Research Grant–Institute for Gender and Health (#CGW- 126580). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Mänty M, Kuh D, Cooper R. Associations of midlife to late life fatigue withphysical performance and strength in early old age: Results from a British prospective cohort study. Psychosom. Med77(7), 823-32 (2015).
- Caldwell JA, Caldwell JL, Schmidt RM. Alertness management strategies for operationalcontexts.Sleep. Med. Rev12(4), 257-273 (2008).
- Connor J, Norton R, Ameratunga S, et al.Driver sleepiness and risk of serious injury to car occupants: population based case control study. BMJ 324(7346), 1125 (2002).
- Stedman’s Medical Dictionary. 27ed, Thomas Lathrop Stedman. Baltimore: Lippincott Williams & Wilkins (2000).
- Kornfeld DS. Consultation-liaison psychiatry: contributions to medical practice. Am. J. Psychiatry159(12), 1964-1972 (2002).
- Ruff RM. Mild traumatic brain injury and neural recovery: rethinking thedebate.NeuroRehabilitation28(3), 167-180 (2011).
- Meares S, Shores EA, Taylor AJ, et al.The prospective course ofpostconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology25(4), 454-465 (2011).
- American Congress of Rehabilitation Medicine: Definition of mild traumatic braininjury.J. Head. Trauma.Rehabil8(1), 86-87 (1993).
- World Health Organization. Neurological disorders: Public health challenges (2010).
- Cassidy JD, Carroll LJ, Peloso PM, et al.for WHO Collaborating Centre Task Force on mild traumatic brain injury. Incidence, risk factors and prevention of mild traumaticbrain injury: results of The WHO Collaboration Centre Task force on Mild Traumatic Brain Injury. J.Rehabi. Med43(suppl), 28-60 (2004).
- Merrick D, Stålnacke BM. Five years post whiplash injury: Symptoms andpsychological factors in recovered versus non-recovered. BMC. Res. Notes3(1), 190 (2010).
- Maroon JC, Mathyssek C, Bost J. Cerebral concussion: a historical perspective. Prog.Neurol.Surg28(1), 01-13 (2014).
- Kraus JF,Hsu P, Schafer K, et al.Sustained outcomes following mild traumatic brain injury: results of a five-emergency department longitudinal study. Brain.Inj28(1), 1248-1256 (2014).
- Katz DI, Cohen SI, Alexander MP. Mild traumatic brain injury.Handb. Clin.Neurol127(1), 131-156 (2015).
- Mollayeva T, Kendzerska T, Mollayeva S, et al. Asystematic review of fatigue in patients with traumatic brain injury: the course,predictorsand consequences.Neurosci.Biobehavav. Rev 47(1), 684-716 (2014).
- Beaulieu-Bonneau S, Fortier-Brochu É, Ivers H, et al.Attention following traumatic brain injury: Neuropsychological and driving simulator data, andassociation with sleep, sleepiness, and fatigue. Neuropsychol.Rehabil24(1), 01-23 (2015).
- Chaumet G, Quera-Salva MA, Macleod A, et al. Isthere a linkbetween alertness and fatigue in patients with traumatic brain injury?Neurol71(20), 1609-1313 (2008).
- Mollayeva T, Mollayeva S, Shapiro CM, et al.Insomnia in workers with delayed recovery from mild traumatic brain injury. Sleep. Med(2015).
- Beaulieu-Bonneau S, Ouellet MC. Fatigue in the first year after traumatic brain injury:course, relationship with injury severity, and correlates.Neuropsychol. Rehabil1-19 (2016).
- Ruff RM, Iverson GL, Barth JT, et al.NAN Policy Planning Committee, Recommendations for diagnosing a mild traumatic brain injury: a national academy of neuropsychology education paper. Arch. Clin.Neuropsychol24(1), 3-10 (2009).
- Mollayeva T, Kendzerska T, Colantonio A. Self-report instruments for assessing sleepdysfunction in an adult traumatic brain injury population: a systematic review.Sleep. Med. Rev17(6), 411-423 (2013).
- Collins GS, Reitsma JB, Altman DG, et al.Transparent Reporting of aMultivariable Prediction Model for Individual Prognosis Or Diagnosis (TRIPOD):theTRIPOD statement.J. Clin.Epidemiol68(1), 134-143 (2015).
- Xiong C, Martin T, Sravanapudi A, et al.Factors associatedwith return to work in men and women with work-related traumatic brain injury. Disabil. Health. J(2015).
- American Psychiatric Association. Diagnostic and statistical manual of mentaldisorders.Text Revision. 4th ed. Washington, DC: American Psychiatric Association (2000).
- Krupp LB, LaRocca NG, Muir-Nash J, et al.The fatigue severityscale.Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch.Neurolog46(10), 1121-1123 (1989).
- Shapiro CM, Auch C, Reimer M, et al. A new approach to the construct of alertness.J.Psychosom. Res60(6), 595-603 (2006).
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep14(1), 50-55 (1991).
- Casson RJ, Farmer LD. Understanding and checking the assumptions of linear regression: a primer for medical researchers. Clin. Exp. Ophthalmol42(1), 590- 596 (2014).
- Mansfield ER, Helms BP. Detecting multicollinearity. Am. Stat36(3), 158-160 (1982).
- Little RJA, Rubin DB. Statistical analysis with missing data. 2ndEdn. Hoboken NJ, Wiley (2002).
- Austin PC, Steyerberg EW. The number of subjects per variable required inlinear regression analyses. J. Clin.Epidemiol68(6), 627-636 (2015).
- Norrie J, Heitger M,Leathem J, et al.Mild traumatic brain injury and fatigue: a prospective longitudinal study. Brain.Inj24(13-14), 1528 (2010).
- Wilk JE., Herrell RK, Wynn GH, et al.Mild traumatic brain injury(concussion), posttraumatic stress disorder, and depression in U.S. soldiers involvedincombat deployments: association with postdeployment symptoms.J.Psychosom. Med74(3), 249-257 (2012).
- Kong W, Lin W, Babiloni F, Hu S, et al.Investigating driver fatigue versus alertness using the Granger causality network. Sensors (Basel)15(8), 19181-19198 (2015).
- Johns MW. Sleep propensity varies with behavior and the situation in which itis measured: the concept of somnificity. J. Sleep. Res11(1), 61-67 (2002).
- Simpson S Jr, Tan H, Otahal P, et al. Anxiety, depression and fatigue at 5-year review following CNS demyelination.Acta. Neurol. Scand(2016).
- McBeth J, Tomenson B, Chew-Graham CA, et al. Common and unique associated factors for medically unexplained chronicwidespread pain and chronic fatigue.J.Psychosom. Res79(6), 484-490 (2015).
- Wu S, Mead G, Macleod M, et al.Model of understanding fatigue afterstroke.Stroke46(3), 893-898 (2015).
- Goerke M, Cohrs S, Rodenbeck A, et al.Differential effect of an anticholinergicantidepressant on sleep-dependent memory consolidation.Sleep 37(5), 977-985 (2014).
- Cottingham C, Ferryman CJ, Wang Q. α2 Adrenergic Receptor Trafficking as aTherapeutic Target in Antidepressant Drug Action.Prog. Mol. Biol. Transl.Sci132(1), 207-225 (2015).
- Fischer CW,Elfving B,Lund S,et al.Behavioral and systemic consequencesof long-term inflammatory challenge. J.Neuroimmunol288(1), 40-46 (2015).
- Antonioli M, Rybka J, Carvalho LA. Neuroimmune endocrine effects ofantidepressants.Neuropsychiatr. Dis. Treat 8(1), 65-83 (2012).
- Schmidt MH. The energy allocation function of sleep: a unifying theory of sleep,torpor, and continuous wakefulness. Neurosci.Biobehav. Rev47(1), 122-153 (2014).
- Gander PH,Mulrine HM, van den Berg MJ,et al.Effects of sleep/wake history and circadian phase on proposed pilot fatigue safety performance indicators. J. Sleep. Res24(1), 110-119 (2015).
- Gander PH,Mulrine HM, van den Berg MJ,et al.Pilot fatigue: relationships with departure and arrival times, flight duration, and direction. Aviat. Space. Environ. Med85(8), 833-840 (2014).
- Akerstedt T,Axelsson J, Lekander M,et al. Do sleep, stress, and illness explain daily variations in fatigue? A prospective study. J.Psychosom. Res76(4), 280-285 (2014).
- Heitger MH,Jones RD, Anderson TJ. A new approach to predicting postconcussion syndrome after mild traumatic brain injury based upon eye movement function.Conf. Proc. IEEE. Eng. Med. Biol.Soc2008(1), 3570-3573 (2008).
- Auvergne L, Bortsov AV, Ulirsch JC, et al. Association of epidemiologic factors andgenetic variants influencing hypothalamic-pituitary-adrenocortical axis function withpostconcussive symptoms after minor motor vehicle collision.Psychosom. Med78(1), 68-78 (2016).
- Lockley SW, Barger LK, Ayas NT, et al.Effects of health care provider work hours and sleep deprivation on safety and performance. Jt. Comm. J. Qual. Patient. Safety33(11 Suppl), 07-18 (2007).
- Heaton KJ, Maule AL, Maruta J, et al. Attention and visual trackingdegradation during acute sleep deprivation in a military sample.Aviat. Space. Environ. Med85(5), 497-503 (2014).
- Blundell S, Ray KK, Buckland M, et al.Chronic fatigue syndrome and circulatingcytokines: A systematic review.Brain.Behav.Immun50(1), 186-195 (2015).
- McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-item short-form health survey (SF- 36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 31(1), 247-263 (1993).
- Mollayeva T, Mollayeva S, Colantonio A. Persons at risk for sleep disorders aftermild traumatic brain injury. Curr. Neurol.Neurosci. Rep16(6), 55 (2016).
- Hoffer ME, Gottshall KR, Mooere R, et al.Characterizing and treating dizzinessafter mild head trauma. Otol.Neurol25(1), 135-138 (2004).
- Suh M, Basu S, Kolster R,et al.Increased oculomotor deficits during target blankingas an indicator of mild traumatic brain injury. Neurosci.Lett410(1), 203-237 (2006).
- Giordano C, Kleiven S. Evaluation of axonal strain as a predictor for mildtraumatic brain injuries using finite element modeling. Stapp. Car. Crash. J58(1), 29-61 (2014).
- Wijemanne S, Jankovic J. Restless legs syndrome: clinical presentation diagnosisandtreatment.Sleep. Med 16(6), 678-690 (2015).
- Kim JB, Koo YS, Eun MY, et al.Psychosomatic symptom profiles inpatients with restless legs syndrome.Sleep.Breath17(3), 1055-1061 (2013).
- Beijamini F, Silva AGT, Peixoto CAT, et al.Influence of gender on psychomotor vigilance task performance by adolescents. Braz. J. Med. Biol. Res41(8), 734-738 (2008).
- Blatter K, Graw P, Münch M, et al. Gender and age differences in psychomotor vigilance performance under differential sleeppressure conditions. Behav. Brain. Res168(2), 312-317 (2006).
- Robertson IH, Manly T, Andrade J, et al.Oops!':performance correlates of everyday attentional failures in traumatic brain injured and normalsubjects. Neuropsychologia35(6), 747-758 (1997).
- Adan A,Sánchez-Turet M. Gender differences in diurnal variations of subjective activation and mood.Chronobiol. Int18(3), 491-502 (2001).
- Axelsson J. Kecklund G, Akerstedt T, et al.Sleepiness and performance in responseto repeatred sleep restriction and subsequent recovery during semi-laboratory conditions. Chronobiology.Internat25(1), 297-308 (2008).
- Spaeth AM, Dinges DF, Goel N. Phenotypic vulnerability of energy balance responses tosleep loss in healthy adults.Sci. Rep5(1), 14920 (2015).
- Chua EC, Yeo SC, Lee IT, et al.Individualdifferences in physiologic measures are stable across repeated exposures to total sleepdeprivation.Physiol. Rep2(9) (2014).
- Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep15(1), 376-431 (1992).
- Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth Sleepiness Scale: failure of theMSLT as a gold standard. J. Sleep. Res9(1), 5-11 (2000).
- Nardone R, Bergmann J, Kunz A, et al.Cortical excitability changes in patients with sleep-wake disturbances after traumatic brain injury. J.Neurotrauma28(7), 1165-1171 (2011).
- Gottlieb DJ, Whitney SW, Bonekat WH, et al.Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am. J.Respir. Crit. Care. Med159(1), 502-507 (1999).
- Melamed S, Oksenberg A. Excessive sleepiness and risk of occupational injuries in non- shift daytime workers. Ann. Med.Milit.Fenn57(suppl), 96 (1982).
- Hesselbacher S, Subramanian S, Allen J, Body mass index, gender, and ethnic variations alter the clinical implications of the Epworth Sleepiness Scale in patients with suspected obstructive sleep apnea. Open.Respir. Med. J6(1), 20-27 (2012).
- Kendzerska TB,Smith PM, Brignardello-Petersen R, et al.Evaluation of the measurement properties of the Epworth sleepiness scale: asystematic review. Sleep. Med. Rev18(4), 321-331 (2014).
- Sarver J, Baker DW. Effect of language barriers on follow-up appointments afteran emergency department visit. J. Gen. Intern. Med15(4), 256-264 (2000).
- Linguistic Characteristics of Canada (2016).
- Facts and figures. 2014 - Immigration overview: Permanent residents (2016).
- Centofanti SA, Dorrian J, Hilditch CJ, et al.Do night naps impact drivingperformance and daytime recovery sleep?Accid. Anal.Prev(2015).
- Devine JK, Wolf JM. Integrating nap and night-time sleep into sleep patterns reveals differential links to health- relevant outcomes. J. Sleep. Res(2015).
- Hamilton JC, Eger M, Razzak S, et al.Somatoform,factitious, and related diagnoses in the national hospital discharge survey: addressingtheproposed DSM-5 revision.Psychosomatics54(2), 142-148 (2013).
- FrostholmL, Petrie KJ, Ornbøl E, et al.Are illness perceptions related to futurehealthcare expenditure in patients with somatoform disorders?Psychol. Med44(13), 2903-2911 (2014).
- Mollayeva T, Thurairajah P, Burton K, et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep. Med. Rev25(1), 52-73 (2016).
- Richardson GS, Drake CL, Roerhs TA, et al.Habitual sleep time predicts accuracyof self-reported alertness. Sleep25(1), S145 (2002).
- Ciccone MM, Aquilino A, Cortese F, et al.Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes (Project Leonardo). Vasc. Health. Risk. Manag 6(1), 297-305 (2010).