Review Article - Neuropsychiatry (2016) Volume 6, Issue 5
Adenosine dysfunction in Rasmussens encephalitis
- Corresponding Author:
- Tianfu Li, M.D., Ph D
Professor and Chief Physician in Neurology, Vice director of Brain Institute, Beijing Sanbo Brain Hospital, Capital Medical University, Xiangshan Yikesong 50, Haidian district, Beijing, 100093, China
Tel: +86 1062856761
Fax: +86-10-62856902
Abstract
Rasmussen’s encephalitis (RE) is neurological disorder of childhood characterized by uni-hemispheric inflammation, intractable focal epilepsy and progressive cognitive and neurological deficits. Currently, hemispherectomy is the only effective method to control the seizures associated with RE. Although this disease has been heavily investigated, the pathogenesis of RE with unilateral cortex atrophy and focal seizure is still enigmatic. Overexpression of the ADK, the major adenosine removing enzyme, was observed in the lesions of RE. As the upper neuromodulator of the brain, adenosine is well known with anti-inflammtion, aniti-epilepsy as well as improving cognitive dysfunction associated with epilepsy. Overexpression of ADK and resulting adenosine deficiency is involved in the development of RE- pharmacoresistant seizures, inflammation, and deficits in cognitive function. Dysregulation of adenosine signaling is a common pathologic hallmark of RE, which suggest the specific targets in the treatment of epilepsy, inflammation and cognitive deterioration associated with epilepsy in RE patients.
Keywords
Rasmussen encephalitis, Adenosine, Epilepsy, Inflammation, Cognition
Introduction
Rasmussen encephalitis (RE) is a very rare chronic progressive inflammatory neurological disorder of uncertain etiology affecting mostly children and associated with hemispheric atrophy, pharmacoresistant focal epilepsy (epilepsia partialis continua), cognitive deterioration and progressive neurological deficits, resulting from progressive loss of function subserved by the involved cerebral hemisphere [1-4]. The aetiology and pathogenesis of RE, in particular, the factors responsible for the characteristic of asymmetry are still unclear. Seizures are a prominent clinical features of RE, while the inflammation plays a crucial role in the pathomechanism of epileptogenesis [5], and clinically comorbid cognition deficits are among the most debilitating and persistent concerns of chronic epilepsy associated with RE. Overexpression of ADK and resulting adenosine deficiency in sclerotic lesion tissue of the RE brain can be an important factor for the development of pharmacoresistant focal seizure, inflammation and cognitive deterioration [6]. Therefore, focal augmentation of adenosine may be an ideal therapeutic strategy for RE with the role of anti-seizure, anti-inflammation and improve the cognitive deterioration. A1R and A2AR might be involved in the therapy of focal augmentation of adenosine for RE. Dysregulation of A1R signaling is intricately linked to the pathophysiology of epilepsy and decreased A1R expression may contribute to seizure generation in human chronic epilepsy [7-9]. Anti-inflammatory actions and cognitive enhancement is mediated by the A2A receptor subtype [10-12], yet this receptor may have a deleterious effect in epilepsy whereas the anti-epileptic action of the A1 receptor is preponderant. In the following we will highlight the reasonable mechanistic explanations how adenosine deficiency might functionally be linked to the development of epilepsy, inflammation and cognition deficits in RE.
▪ Adenosine dysfunction and epilepsy
Extensive evidence demonstrated that adenosine is an inhibitory modulator of brain activity, and its anticonvulsant and seizure terminating effects, mediated by both receptor-dependent and receptor–independent pathways, have been illustrated in experimental models of epilepsy [9,13].
▪ Adenosine receptor-dependent pathway
Neuronal excitability in the brain is modulated by activation of G protein coupled adenosine receptors (A1, A2A, A2B, A3) [14,15]. The receptor expression levels and availability of endogenous adenosine to activate the receptors plays a crucial role in neuronal excitability [9]. Imbalance of adenosine receptor activation (decreased A1R signaling and increased A2AR signaling) contributes to the pathophysiology and development of epilepsy. Endogenous adenosine acting at A1R is an important seizure-control mechanism. Currently increased expression of adenosine kinase (ADK)-the main adenosineremoving enzyme, and decreased A1R signaling, both contributing to reduce the adenosine tone, are regarded as important factor contributing to the development and pathophysiology of epilepsy as well as a potential target for anti-epileptogenesis or disease modification [6,9,16-25]. A1R are enriched in the central nervous system, where they are expressed in the cerebral cortex, hippocampus, cerebellum, thalamus, and brainstem. In the brain, adenosine modulates neuronal activity by decreasing presynaptic release of various neurotransmitters, and the most dramatic inhibitory actions are on the glutamatergic system [26]. In addition, adenosine acting through postsynaptic A1R may activate K+ channels, leading to hyperpolarization of postsynaptic neurons and promoting NMDA receptor inhibition [27]. Deletion of A1R or increased adenosine clearance by overexpression of ADK (which should reduce A1R activation) both cause spontaneous electrographic seizures [18,19,22] and develop lethal status epilepticus following the intrahippocampal injection of kainic acid in rodent models of epilepsy [8]. The ability of adenosine to prevent or ameliorate seizures induced by pentylenetetrazole, pilocarpine, NMDA, bicuculline, organophosphate treatment, and electrical stimulation has been attributed essentially to A1R activation, which inhibits presynaptic excitatory neurotransmitter release and hyperpolarises the postsynaptic cell membrane [28]. In addition to the several lines of experimental animal research that supports the important anticonvulsant role of adenosine, increasing clinical evidence from specimen surgically resected from patients with pharmacoresistant epilepsy also demonstrated that adenosine dysfunction contributing to seizure generation in human chronic epilepsy, including i) adenosine deficiency in microdialysis samples from epileptogenic hippocampus in human patients with TLE [29]; ii) overexpression of astroglial ADK within the epileptic foci in temporal lobe epilepsy [22,30]; Rasmussen encephalitis [6], astroglial tumor-related epilepsy [31] and focal cortical dysplasia [21], leading to decrease the adenosine level and A1R activation; iii) genetic variation in ADK associating with posttraumatic epilepsy development and contributing to explaining variability in time to first seizure and posttraumatic epilepsy risk, indicating that genetic variation in adenosine regulatory pathways relating to epileptogenesis and ADK may be the therapeutic targets for pharmacotherapy development [32]; iv) variants in the A1R gene associating with the development of posttraumatic seizures after a severe traumatic brain injury and indicating that deficiency in A1R signaling might be associated with posttraumatic epileptogenesis; v) loss of A1R in human temporal lobe epilepsy, demonstrating that loss of anticonvulsant A1R may contribute to the human epileptic condition [7]. The reason for the A1R loss (in the epileptic human brains) is unclear as it occurred in both idiopathic and symptomatic cases and thus may be a consequence rather than an initial cause of seizures. It is also possible that the observed differences in A1 binding are due to autopsy vs. biopsy changes in the levels of A1R [7]. On the other hand, the epileptic state is indeed characterized by decreased A1R signaling and increased A2AR signaling and low adenosine levels increase the amount of excitatory A2A receptors (the second most abundant adenosine receptor) in the brain of drug-resistant epileptic patients. A2AR knockout mice were partially resistant to limbic seizures [33], and activation of central adenosine A(2A) receptors lowers the seizure threshold of hyperthermia-induced seizure in childhood rats [34].
▪ Adenosine receptor-independent pathway
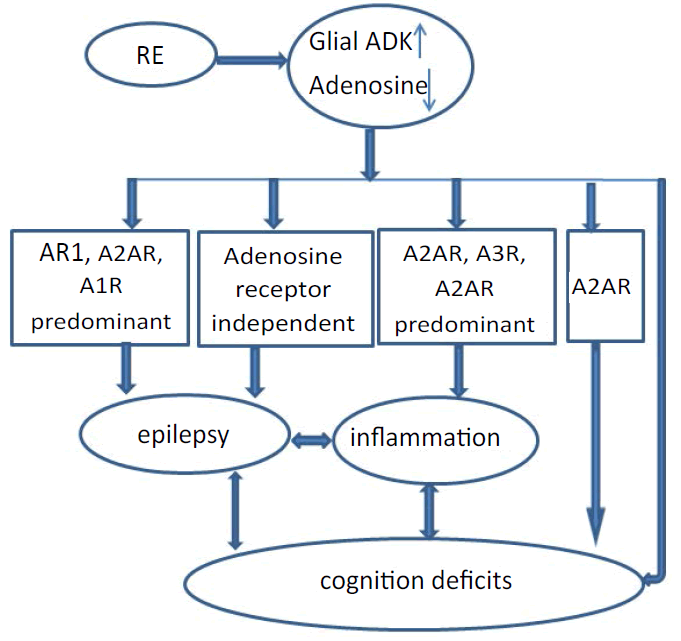
In addition to adenosine receptor dependent effects, adenosine exerts receptor-independent effects in DNA methylation homeostasis [9,13]. Adenosine is an obligatory end product of S-adenosylmethionine (SAM) dependent transmethylation reactions, which also include methyl group transfers onto DNA, catalyzed by DNA methyltransferases [9,13]. Increased ADK expression drives an increase in the transmethylation pathway leading to hypermethylated DNA, which is potentially implicated in epileptogenesis [35]. Increased ADK and increased DNA methylation status form a vicious cycle implicated in the progression and maintenance of the epileptic state. Therefore, dysregulation of ADK plays a significant role in the processes that turn a normal brain into an epileptic brain. This would seem to suggest the use of ADK inhibitors in epilepsy therapy. However, the chronic systemic use of ADK inhibitors might not be a viable therapeutic option due to liver toxicity, and the occurrence of brain hemorrhage in some of the preclinical studies as well as cognitive and sedative adverse effects [36]. Gene therapy directed to ADK through an antisense oligonucleotide as a means of conserving adenosine by reducing ADK expression has been investigated [37,38]. Adenosine-releasing polymer implanted to the brain ventricles of epileptic rats demonstrated that focal augmentation of adenosine restores normal DNA methylation and thereby prevents epileptogenesis [13]. The mechanism of action of Ketogenic diet (KD), an often prescribed protocol to treat pediatric pharmacoresistant epilepsy, involved adenosine receptor-dependent pathway and adenosine receptor-independent pathway (Figure 1). On the one hand, KD downregulates ADK to increase adenosine levels in the brain to enhance adenosine signaling and A1R activation [22,39]. On the other hand, KD treatment has been shown to increase adenosine levels and exerts receptor-independent effects in DNA methylation homeostasis to reduce DNA methylation [13,40]. There is every indication that agents able to increase adenosine availability may have a place in the future treatment of epilepsy via adenosine receptor-dependent pathway and adenosine receptor-independent pathway [41].
▪ Adenosine dysfunction and inflammation
Adenosine is an endogenous purine nucleoside that modulates a wide range of physiological functions [7]. Most notable among its many roles is its importance in controlling inflammation [42,43] and inhibiting seizures [9]. Homeostasis of adenosine receptor signaling is of crucial importance in the regulation of inflammation and the release of proinflammatory cytokines release from macrophages, dendritic cells, and lymphocytes [44-47]. It is well accepted that adenosine exerts potent anti-inflammatory effects via activation of A2A and A3R (Figure 1), and that A2A and A3R agonists potentially having a relevant role in the treatment of rheumatoid arthritis [41]. The A2A and A3R in particular play key roles in the regulation of inflammatory pathways in a variety of conditions including arthritis. Activation of A2AR prevented the collagen-induced arthritis progression by preventing nitrosative and oxidative injury and reducing the levels of cytokines such as TNFα, interleukin (IL)1β [48], there by suggesting a role for A2A receptors in inflammation. Also adenosine was found to suppress elevated levels of the proinflammatory cytokines TNFα and IL-1β in patients with rheumatoid arthritis [49]. A2BR subtype is selectively induced in inflamed vascular and intestinal epithelia, as well as the kidneys, heart and lung, making it a direct target in the treatment of inflammation characterized by tissue hypoxia [50].
The proposed anti-inflammatory properties of adenosine are most frequently mediated via A2A (and A3, less abundant in the brain) receptors, but only indirect evidences have been provided that this pathway is activated in the brain of RE patients.
▪ Adenosine dysfunction and cognition deficits
Apart from mediating seizure inhibition and inflammation control, adenosine is a crucial regulator of behavior and disruption of adenosine homeostasis has been linked with cognitive and psychiatric phenotypes [9]. Adenosine, a key upstream modulator of major neurotransmitter systems including glutamatergic and GABAergic neurotransmission [51,52], provides a crucial role in the regulation of cognitive processes [36]. ADK is the primary route of adenosine metabolism in brain and minor changes in ADK activity translate rapidly into major changes in adenosine. Thereby, dysregulation of ADK expression and resulting disruption of adenosine homeostasis is implicated in a wide range of neurologic and neuropsychiatric pathologies. The link between overexpression of ADK and cognitive impairment might be of pathologic relevance for neurologic conditions in which overexpression of ADK has been confirmed in epilepsy: i) Transgenic overexpression of ADK in the brain of mice (Adk-tg mice) caused prominent cognitive impairment on several levels [53], and Adk-tg mice displayed severe learning deficits in the domains of reference memory, working memory, and associative learning, in particular severe learning deficits in the Morris water maze task and in Pavlovian conditioning [54]. ii) Adenosine releasing cell grafts to the hippocampal formation to reconstruct of adenosine homeostasis demonstrate cognitive performance improving in Adk transgenic mice [55]; iii) Astrogliosis-associated overexpression of ADK might be causally involved in the development of cognitive comorbidities in pharmacoresistant epilepsy such as temporal lobe epilepsy [22,30], focal cortical dysplasia [21], Rusmussen encephalitis [6]; iv) Astroglial A2A receptor affects cognitive function through a novel mechanism involving astrocyte-driven neuronal adaptation processes (Figure 1). The effects of A2AR on memory are heralded by the ability of A2AR to impact working memory [10,11,56,57] and especially reference memory performance [56], and the pharmacological or genetic blockade of A2AR impedes memory deterioration [57]. Via controlling astrocytic glutamate transporter-I activity, dysfunction of astrocytic A2AR, triggers an astrocyte-to-neuron wave of communication resulting in disrupted glutamate homeostasis [58].
Outlook and conclusions
Adenosine signalling may be dysregulated in the brain of patients with RE and that this pathological feature might explain unihemispheric inflammation, intractable focal epilepsy and progressive cognitive and neurological deficits in these patients. Adenosine system (and/or adenosine manipulation via its major metabolic enzyme ADK) could be of potential importance of therapeutic value against RE. Currently no direct evidences about adenosine receptors activation pathway have been provided in the brain of RE patients. More research needs to be done including identification of the characteristics of adenosine kinase and adenosine receptors expression including the density, properties, localization and function in brain at different stage of clinical course of RE patients. Understanding these mechanisms might eventually develop entirely new conceptual strategies to treat the complex comorbid syndrome of RE.
Acknowledgment
This Project was supported by the Grant from the BIBD-PXM2013_014226_07_000084, National Natural Science Foundation of China (81571275), Scientific Research Common Program of Beijing Commission of Education (KM201410025027). We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Competing interests
The authors declare that they have no competing interests.
References
- Rasmussen T, Olszewski J, Lloydsmith D. Focal seizures due to chronic localized encephalitis. Neurology8(6), 435-445 (1958).
- Bien CG, Granata T, Antozzi C, et al.Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain128(1), 454-471 (2005).
- Bien CG, Widman G, Urbach H, et al.The natural history of Rasmussen's encephalitis. Brain 125(), 1751-1759 (2002).
- Varadkar S, Bien CG, Kruse CA, et al.Rasmussen's encephalitis: clinical features, pathobiology, and treatment advances. Lancet. Neurol13(2), 195-205 (2014).
- Luan G, Gao Q, Zhai F, et al.Upregulation of HMGB1, toll-like receptor and RAGE in human Rasmussen's encephalitis. Epilepsy. Res123(1), 36-49 (2016).
- Luan G, Gao Q, Guan Y, et al.Upregulation of adenosine kinase in Rasmussen encephalitis. J.Neuropathol. Exp. Neurol72(11), 1000-1008 (2013).
- Glass M, Faull RL, Bullock JY, et al.Loss of A1 adenosine receptors in human temporal lobe epilepsy. Brain. Res710(1-2), 56-68 (1996).
- Fedele DE, Li T, Lan JQ, et al.Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp. Neurol200(1), 184-190 (2006).
- Boison D. Adenosinergic signaling in epilepsy. Neuropharmacology104(1), 131-139 (2016).
- Chen JF. Adenosine receptor control of cognition in normal and disease. Int. Rev. Neurobiol119(1), 257-307 (2014).
- Hu Q, Ren X, Liu Y, et al. Aberrant adenosine A2A receptor signaling contributes to neurodegeneration and cognitive impairments in a mouse model of synucleinopathy. Exp. Neurol283(1), 213-223 (2016).
- Madeira MH, Boia R, Elvas F, et al.Selective A2A receptor antagonist prevents microglia-mediated neuroinflammation and protects retinal ganglion cells from high intraocular pressure-induced transient ischemic injury. Transl. Res169(1), 112-128 (2016).
- Williams-Karnesky RL, Sandau US, Lusardi TA, et al.Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J. Clin. Invest123(8), 3552-3563 (2013).
- Fredholm BB, Chen JF, Masino SA et al.Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol45(1), 385-412 (2005).
- Fredholm BB, IJzerman AP, Jacobson KA, et al.International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors-an update. Pharmacol. Rev63(1), 01-34 (2011).
- Li T, Ren G, Kaplan DL et al.Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3-selective epileptogenesis. Epilepsy. Res84(2-3), 238-241 (2009).
- Boison D. Adenosine dysfunction in epilepsy. Glia60(8), 1234-1243 (2012).
- Li T, Lytle N, Lan JQ, et al.Local disruption of glial adenosine homeostasis in mice associates with focal electrographic seizures: a first step in epileptogenesis? Glia60(1), 83-95 (2012).
- Li T, Ren G, Lusardi T, et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J. Clin. Invest118(2), 571-582 (2008).
- Li T, Steinbeck JA, Lusardi T, et al.Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain130(5), 1276-88 (2007).
- Luan G, Gao Q, Zhai F, et al.Adenosine kinase expression in cortical dysplasia with balloon cells: analysis of developmental lineage of cell types. J. Neuropathol. Exp. Neurol74(2), 132-147 (2015).
- Masino SA, Li T, Theofilas P, et al.A ketogenic diet suppresses seizures in mice through adenosine A(1) receptors. J. Clin. Invest121(7), 2679-83 (2011).
- Wilz A, Pritchard EM, Li T, et al.Silk polymer-based adenosine release: therapeutic potential for epilepsy. Biomaterials29(26), 3609-3916 (2008).
- Li T, Quan LJ, Fredholm BB, et al.Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron. Glia. Biol3(4), 353-366 (2007).
- Li T, Lan JQ, Boison D. Uncoupling of astrogliosis from epileptogenesis in adenosine kinase (ADK) transgenic mice. Neuron. Glia. Biol4(2), 91-99 (2008).
- Reppert SM, Weaver DR, Stehle JH, et al.Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol. Endocrinol5(8), 1037-1048 (1991).
- Wardas J. Neuroprotective role of adenosine in the CNS. Pol. J. Pharmacol54(4), 313-326 (2002).
- Masino SA, Kawamura MJ, Ruskin DN. Adenosine receptors and epilepsy: current evidence and future potential. Int. Rev. Neurobiol119(1), 233-255 (2014).
- During MJ and Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann. Neurol32(5), 618-624 (1992).
- Aronica E, Zurolo E, Iyer A, et al.Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia52(9), 1645-1655 (2011).
- de Groot M, Iyer A, Zurolo E, et al.Overexpression of ADK in human astrocytic tumors and peritumoral tissue is related to tumor-associated epilepsy. Epilepsia53(1), 58-66 (2012).
- Diamond ML, Ritter AC, Jackson EK, et al.Genetic variation in the adenosine regulatory cycle is associated with posttraumatic epilepsy development. Epilepsia56(8), 1198-1206 (2015).
- El YM, Ledent C, Parmentier M, et al.Adenosine A2A receptor deficient mice are partially resistant to limbic seizures. Naunyn. Schmiedebergs. Arch. Pharmacol380(3), 223-232 (2009).
- Fukuda M, Suzuki Y, Hino H, et al. Activation of central adenosine A(2A) receptors lowers the seizure threshold of hyperthermia-induced seizure in childhood rats. Seizure20(2), 156-159 (2011).
- Kobow K and Blumcke I. The emerging role of DNA methylation in epileptogenesis. Epilepsia 53(9), 11-20 (2012).
- Boison D. Adenosine kinase: exploitation for therapeutic gain. Pharmacol. Rev65(3), 906-943 (2013).
- Boison D and Aronica E. Comorbidities in Neurology: Is adenosine the common link? Neuropharmacology97(1), 18-34 (2015).
- Theofilas P, Brar S, Stewart KA, et al. Adenosine kinase as a target for therapeutic antisense strategies in epilepsy. Epilepsia52(3), 589-601 (2011).
- Greene RW. Adenosine: front and center in linking nutrition and metabolism to neuronal activity. J. Clin. Invest121(7), 2548-2550 (2011).
- Lusardi TA, Akula KK, Coffman SQ, et al.Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology99(1), 500-509 (2015).
- Borea PA, Gessi S, Merighi S, et al.Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends. Pharmacol. Sci37(6), 419-434 (2016).
- Mills JH, Kim DG, Krenz A, et al.A2A adenosine receptor signaling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis. J. Immunol 188(11), 5713-5722 (2012).
- Blackburn MR, Vance CO, Morschl E et al.Adenosine receptors and inflammation. Handb. Exp. Pharmacol193(1), 215-269 (2009).
- Antonioli L, Csoka B, Fornai M, et al. Adenosineand inflammation: what's new on the horizon? Drug. Discov. Today19(8), 1051-1068 (2014).
- Hasko G and Pacher P. Regulation of macrophage function by adenosine. Arterioscler. Thromb. Vasc. Biol32(4), 865-869 (2012).
- Wei W, Du C, Lv J, et al.Blocking A2B adenosine receptor alleviates pathogenesis of experimental autoimmune encephalomyelitis via inhibition of IL-6 production and Th17 differentiation. J. Immunol190(1), 138-146 (2013).
- Perretti M, Leroy X, Bland EJ, et al.Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation. Trends. Pharmacol. Sci36(11), 737-755 (2015).
- Mazzon E, Esposito E, Impellizzeri D, et al.CGS 21680, an agonist of the adenosine (A2A) receptor, reduces progression of murine type II collagen-induced arthritis. J. Rheumatol 38(10), 2119-2129 (2011).
- Varani K, Padovan M, Vincenzi F, et al.A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis. Res. Ther13(6), R197 (2011).
- Poth JM, Brodsky K, Ehrentraut H, et al. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. J. Mol. Med (Berl) 91(2), 183-193 (2013).
- Diogenes MJ, Neves-Tome R, Fucile S, et al.Homeostatic control of synaptic activity by endogenous adenosine is mediated by adenosine kinase. Cereb. Cortex24(1), 67-80 (2014).
- Rombo DM, Dias RB, Duarte ST, et al.Adenosine A1 Receptor Suppresses Tonic GABAA Receptor Currents in Hippocampal Pyramidal Cells and in a Defined Subpopulation of Interneurons. Cereb. Cortex26(3), 1081-1095 (2016).
- Singer P, McGarrity S, Shen HY, et al.Working memory and the homeostatic control of brain adenosine by adenosine kinase. Neuroscience213(1), 81-92 (2012).
- Yee BK, Singer P, Chen JF, et al.Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. Eur. J. Neurosci26(11), 3237-3252 (2007).
- Shen HY, Singer P, Lytle N, et al.Adenosine augmentation ameliorates psychotic and cognitive endophenotypes of schizophrenia. J. Clin. Invest122(7), 2567-2577 (2012).
- Cunha RA and Agostinho PM. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J. Alzheimers. Dis 20(1), S95-116 (2010).
- Cunha RA. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem(2016).
- Matos M, Shen HY, Augusto E, et al.Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol. Psychiatry78(11), 763-774 (2015).