Research Article - Neuropsychiatry (2016) Volume 6, Issue 6
Abnormal developmental trajectories of amplitude of low frequency fluctuations within intrinsic connectivity networks for school-age boys with ADHD
- Corresponding Author:
- Xun-Heng Wang, Ph.D
College of Life Information Science and Instrument Engineering, Hangzhou Dianzi University, Hangzhou, 310018, China
Phone: 0571-87713533
Fax: 0571-87713528
Abstract
Background:
ADHD is a prevalent brain disorder in school-age children. To our knowledge, the development of intrinsic connectivity networks (ICNs) remains unclear for children with ADHD. The goals of this paper are two-folds: 1) modeling the ICN-related brain connectivity based on phenotype scores; 2) exploring the altered growth curves of ICNs for ADHD.
Methods and Findings:
A cohort of boys with ADHD and a cohort of normal controls were recruited from ADHD-200 Consortium. Amplitude of low frequency fluctuations (ALFFs) was applied to measure the brain connectivity within ICNs. Quantic models consisted of age; IQ, behavioral scores and head motion were applied to investigate the relationships between brain developments and intra-ICN ALFFs. The results found that the lateral visual network and executive control network were nonlinearly correlated to aging in both ADHD group and normal control group. Based on intra-ICN ALFFs, the turning points of brain developments might be 11-12 years old for ADHD.
Conclusions:
The lateral visual network, cerebellum network, auditory network, and executive control network might play important roles in the brain development of ADHD. The abnormal developmental trajectories of ADHD could be discovered by intra-ICN ALFFs, which could be a potential biomarker for functional connectome.
Keywords
ADHD, Intrinsic connectivity networks, Temporal patterns, Growth curves, ALFFs
Introduction
ADHD is a common brain disorder observed in school-age children [1]. Structural MRIrelated studies found decreased brain volume and thinning cortical thickness in patients with ADHD [2,3]. Diffusion MRI-related studies found altered white matters in ADHD [4]. Resting-state functional MRI-related studies found patients with ADHD exhibited altered amplitude of low frequency fluctuations (ALFFs) and regional homogeneity (ReHo) [5,6], most of which were located in default mode networkrelated brain regions [7,8].
In addition to the distinctive brain mechanisms in ADHD, the abnormal brain developmental patterns in ADHD have also been reported by many studies. The cerebellar developments measured by volume size were lagged in children and adolescents with ADHD [9]. The maturations of cortical thickness and surface area were both delayed in children with ADHD [10]. Furthermore, growth curves of cortical thickness were related to different IQ levels in children with ADHD [11]. Lagged functional connectivities were found between sensorymotor and default mode-related resting state networks in children with ADHD [12]. Lagged maturations of functional connectivity within default mode network and between certain brain networks were reported by analyzing a large sample of children with ADHD [13]. Therefore, MRI-based measures might be beneficial to investigate brain maturations for ADHD. However, the developmental patterns of largescale brain networks remain unclear for children with ADHD.
Abnormal large-scale brain networks were reported in patients with ADHD [14]. The altered spatial components of default mode network were discovered in ADHD using independent component analysis [15]. However, multivariate spatial features of intrinsic connectivity networks (ICNs) might lead difficulties in fitting the growth curves for patients. Thus, univariate features might benefit the research of ICN-related brain development for ADHD. Recently, the univariate temporal complexity of ICN dynamics was found with good test-retest reliability [16] and could reflect the information flows within brain networks [17]. The above findings suggested that the temporal dynamics of ICN could be potential biomarkers for brain disorders. Therefore, the Univariate temporal patterns of ICN might possess biological meanings in some extent, and could be beneficial for investigations of ADHD development.
Based on previous studies, we hypothesized that children with ADHD, particularly schoolage boys, might have abnormal developmental trajectories of brain networks, which could be detected by temporal complexity within ICNs. To test this hypothesis, ALFFs were used as temporal pattern to analyze the information flow within ICNs for patients and normal controls. Moreover, the relationships between age and intra-ICN ALFFs were analyzed using regression models. The altered developmental trajectories of intra-ICN ALFFs were discovered by comparing ADHD group with normal control group.
Methods
▪ Participants and MRI protocols
A cohort of 80 boys with ADHD (8.33-14.92 years old) and a cohort of 77 normal-developed boys (8-14.92 years old) were recruited from the Peking site of ADHD-200 Consortium [18]. The ethic of this study has been approved by functional 1000 connectome project. All patients and normal controls were diagnosed using the Schedule of Affective Disorders and Schizophrenia for Children-Present and Lifetime Version (KSADS-PL). The clinical symptoms of patients were evaluated with ADHD Rating Scale (ADHD-RS) IV. The Intelligence Quotient (IQ) scores for all participants were measured using Wechsler Intelligence Scale for Chinese Children-Revised (WISCC-R). In addition, the patient group contains three subtypes of ADHD. Of note, certain patients exhibited secondary clinical symptoms (Table 1).
| ICN1 (T, p) |
ICN2 (T, p) |
ICN3 (T, p) |
ICN4 (T, p) |
ICN5 (T, p) |
ICN6 (T, p) |
ICN7 (T, p) |
ICN8 (T, p) |
ICN9 (T, p) |
ICN10 (T, p) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| (Intercept) | (1.07, 0.29) | (1.71, 0.09) | (3.02, 0) | (1.33, 0.19) | (1.92, 0.06) | (1.77, 0.08) | (1.82, 0.07) | (2.44, 0.02) | (-0.75, 0.45) | (1.84, 0.07) |
| age | (-0.8, 0.43) | (-1.4, 0.17) | (-2.71, 0.01) | (-0.98, 0.33) | (-1.97, 0.05) | (-1.76, 0.08) | (-1.8, 0.08) | (-2.2, 0.03) | (1.45, 0.15) | (-1.06, 0.29) |
| age2 | (0.85, 0.4) | (1.48, 0.14) | (2.81, 0.01) | (1.16, 0.25) | (2.09, 0.04) | (1.82, 0.07) | (1.89, 0.06) | (2.24, 0.03) | (-1.2, 0.24) | (1.2, 0.24) |
| IQ | (-0.01, 0.99) | (-0.37, 0.71) | (0.53, 0.59) | (-0.13, 0.9) | (-1.2, 0.23) | (-0.16, 0.88) | (0.75, 0.45) | (-0.2, 0.84) | (0.01, 0.99) | (-1.77, 0.08) |
| Inattention | (-0.91, 0.37) | (-1.23, 0.22) | (-0.34, 0.73) | (-0.28, 0.78) | (2.31, 0.02) | (0.32, 0.75) | (0.45, 0.65) | (1.06, 0.29) | (-0.59, 0.56) | (-0.64, 0.52) |
| Impulsivity | (0.9, 0.37) | (0.89, 0.38) | (1.17, 0.25) | (0.93, 0.36) | (2.01, 0.05) | (1.99, 0.05) | (1.2, 0.24) | (1.65, 0.1) | (0.57, 0.57) | (1.47, 0.15) |
| mFD | (2.12, 0.04) | (2.78, 0.01) | (3.03, 0a) | (5.01, 0a) | (5.37, 0a) | (3.8, 0a) | (2.98, 0a) | (4.25, 0a) | (2.19, 0.03) | (2.41, 0.02) |
Table 1: Subjects’ demographic variables.
Both structural and resting state functional images were collected for each participant. The high-resolution T1-weighted images were obtained using Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequences. The resting state images were obtained using standard echo-planar imaging (EPI) sequences. Participants were instructed to relax and remain still with eyes either open or closed during scan sessions. A black screen with a white cross was presented to each participant during the scan. The detailed information of scan parameters could be found on the website of ADHD-200 Consortium [19].
▪ Preprocessing
The pipeline of data preprocessing was based on FSL [20] and AFNI [21], according to the scripts of functional 1000 connectome project [22]. The structural images were skull-stripped and nonlinearly transformed into MNI brain space. The preprocessing for resting state images contained the following steps: 1) discarded the first five volumes; 2) slice-timing; 3) motion correction; 4) regressing out Friston-24 motion parameters, CSF, whiter matter, global signal, as well as linear and quantic trends; 5) spatial smoothing using Gaussian kernel of FWMH = 6 mm; 6) nonlinearly normalized into MNI space with spatial resolution of 3 mm × 3 mm × 3 mm.
▪ Intra-ICN ALFFs
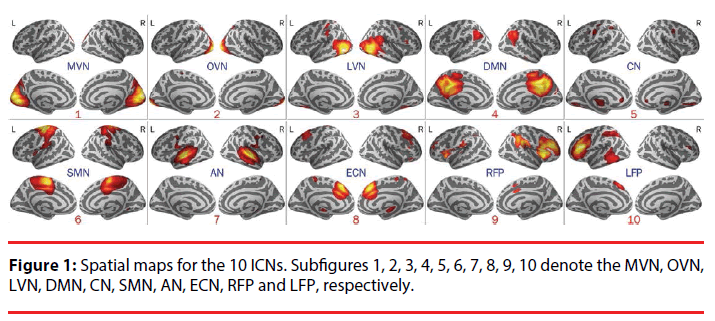
The amplitude of low frequency fluctuations (ALFF) is a popular measure for resting state fMRI [6]. Here, ALFFs were applied to investigate the temporal dynamics within ICNs [23]. Ten well-established templates of ICNs [24] were illustrated in Figure 1 and Table 2. The spatial components of ICNs were consisted of visual, default mode, cerebellum, sensorimotor, auditory, executive control, and frontoparietalrelated networks. For detailed information of the spatial components of ICNs and spatial regression, please see [25] and [16]. First, spatial general linear regressions were applied between the 3-dimentional maps of template ICNs and functional volumes. Then, the timecourses of ICNs were generated by temporally concatenating the beta values returned by spatial regressions. Finally, the intra-ICN ALFFs (0.01- 0.1Hz) were computed by conducting Fast- Fourier Transform (FFT) on the individual time-courses of corresponding ICNs.
| ADHD | Normal | p-value | |
|---|---|---|---|
| Number of subjects | 80 | 77 | - |
| Gender (male: female) | 80:0 | 77:0 | 1 |
| Handless (R: L) | 80:0 | 77:0 | 1 |
| Age (year) | 12.07 ± 1.86 | 11.84 ± 1.8 | 0.44 |
| Full IQ | 107.78 ± 12.82 | 119.31 ± 13.68 | <10-5 |
| Inattentive scores | 49.82 ± 8.89 | 28.95 ± 6.61 | <10-30 |
| Impulsive scores | 28.97 ± 5.56 | 16.75 ± 4.88 | <10-30 |
Table 2: Names of 10 ICNs.
▪ Models for developmental trajectories
Quadratic models were applied to investigate the nonlinear relationships between age and intra-ICN ALFFs. Here, equation 1.1 denoted formula of the quadratic model, where the age, IQ, inattention, impulsivity and head motion might have influences on the intra-ICN ALFFs. In this paper, head motion was measured by mean frame displacement (mFD) [26]. The IQ was indicated by full IQ values. The clinical scores of inattention and impulsivity were measured by ADHD-RS. Finally, the impacts of age on intra-ICN ALFFs were analyzed by regressing out the covariates of IQ, inattention, impulsivity and head motion. The comparisons between ADHD and normal control groups of growth curves were applied by the growthcurve function in the statmod packages [27].
Intra-ICN ALFFs = 1 + age + age2 + IQ + inattention + impulsivity + mFD (1.1)
Results
The developmental trajectories of intra-ICN ALFFs were successfully modeled by phenotype scores. Significant age-related effects on the developmental trajectories of intra-ICN ALFFs were found for certain ICNs. In addition, altered growth curves were found between ADHD group and normal control group. The turning points of brain developmental trajectories were also found for ADHD patients and normal controls.
▪ Effects of age, behavioral scores and head motion on intra-ICN ALFFs for ADHDs
Table 3 shows the effect of age, behavioral scores and head motion on intra-ICN ALFFs for ADHDs. Significant age-related effects are found within lateral visual network, cerebellum and executive control network for ADHD.Significant attention-related effect is found within cerebellum for ADHD. Head motion is related to intra-ICN ALFFs for most of the brain networks. No effects of IQ on intra-ICN ALFFs are found for ADHD.
| ICN1 (T, p) |
ICN2 (T, p) |
ICN3 (T, p) |
ICN4 (T, p) |
ICN5 (T, p) |
ICN6 (T, p) |
ICN7 (T, p) |
ICN8 (T, p) |
ICN9 (T, p) |
ICN10 (T, p) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| (Intercept) | (1.07, 0.29) | (1.71, 0.09) | (3.02, 0) | (1.33, 0.19) | (1.92, 0.06) | (1.77, 0.08) | (1.82, 0.07) | (2.44, 0.02) | (-0.75, 0.45) | (1.84, 0.07) |
| age | (-0.8, 0.43) | (-1.4, 0.17) | (-2.71, 0.01) | (-0.98, 0.33) | (-1.97, 0.05) | (-1.76, 0.08) | (-1.8, 0.08) | (-2.2, 0.03) | (1.45, 0.15) | (-1.06, 0.29) |
| age2 | (0.85, 0.4) | (1.48, 0.14) | (2.81, 0.01) | (1.16, 0.25) | (2.09, 0.04) | (1.82, 0.07) | (1.89, 0.06) | (2.24, 0.03) | (-1.2, 0.24) | (1.2, 0.24) |
| IQ | (-0.01, 0.99) | (-0.37, 0.71) | (0.53, 0.59) | (-0.13, 0.9) | (-1.2, 0.23) | (-0.16, 0.88) | (0.75, 0.45) | (-0.2, 0.84) | (0.01, 0.99) | (-1.77, 0.08) |
| Inattention | (-0.91, 0.37) | (-1.23, 0.22) | (-0.34, 0.73) | (-0.28, 0.78) | (2.31, 0.02) | (0.32, 0.75) | (0.45, 0.65) | (1.06, 0.29) | (-0.59, 0.56) | (-0.64, 0.52) |
| Impulsivity | (0.9, 0.37) | (0.89, 0.38) | (1.17, 0.25) | (0.93, 0.36) | (2.01, 0.05) | (1.99, 0.05) | (1.2, 0.24) | (1.65, 0.1) | (0.57, 0.57) | (1.47, 0.15) |
| mFD | (2.12, 0.04) | (2.78, 0.01) | (3.03, 0a) | (5.01, 0a) | (5.37, 0a) | (3.8, 0a) | (2.98, 0a) | (4.25, 0a) | (2.19, 0.03) | (2.41, 0.02) |
ap<0.01
Table 3: Effects of age, behavioral scores and head motion on intra-ICN ALFFs for ADHDs.
▪ Effects of age, behavioral scores and head motion on intra-ICN ALFFs for normal controls
Table 4 shows the effect of age, behavioral scores and head motion on intra-ICN ALFFs for normal controls. Significant age-related effects are found within lateral visual network, auditory network and executive control network for normal controls. Significant attention-related effect is found within occipital visual network for normal controls. Head motion is related to intra- ICN ALFFs for certain networks. No effects of IQ on intra-ICN ALFFs are found for normal controls.
| ICN1 (T, p) |
ICN2 (T, p) |
ICN3 (T, p) |
ICN4 (T, p) |
ICN5 (T, p) |
ICN6 (T, p) |
ICN7 (T, p) |
ICN8 (T, p) |
ICN9 (T, p) |
ICN10 (T, p) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| (Intercept) | (-0.33, 0.74) | (-0.95, 0.35) | (-2.08, 0.04) | (-1.27, 0.21) | (-0.71, 0.48) | (-0.96, 0.34) | (-2.26, 0.03) | (-1.91, 0.06) | (-0.94, 0.35) | (-1.06, 0.29) |
| age | (0.32, 0.75) | (0.98, 0.33) | (2.59, 0.01) | (1.71, 0.09) | (1.2, 0.23) | (1.33, 0.19) | (2.79, 0.01) | (2.77, 0.01) | (1.46, 0.15) | (1.83, 0.07) |
| age2 | (-0.15, 0.88) | (-0.82, 0.42) | (-2.52, 0.01) | (-1.55, 0.13) | (-1.12, 0.27) | (-1.23, 0.22) | (-2.71, 0.01) | (-2.74, 0.01) | (-1.37, 0.17) | (-1.91, 0.06) |
| IQ | (-0.27, 0.79) | (-0.38, 0.71) | (0.46, 0.65) | (0.35, 0.73) | (-0.38, 0.7) | (0.06, 0.96) | (0, 1) | (0.23, 0.82) | (0.79, 0.43) | (1.26, 0.21) |
| Inattention | (1.49, 0.14) | (2.23, 0.03) | (0.11, 0.91) | (1.83, 0.07) | (0.24, 0.81) | (0.27, 0.79) | (0.05, 0.96) | (-1.75, 0.08) | (0.15, 0.88) | (-0.51, 0.61) |
| Impulsivity | (-0.68, 0.5) | (-0.92, 0.36) | (1.67, 0.1) | (-0.62, 0.54) | (0.68, 0.5) | (0.78, 0.44) | (1.74, 0.09) | (2.02, 0.05) | (0.82, 0.42) | (0.56, 0.58) |
| mFD | (2.3, 0.02) | (2.58, 0.01) | (1.24, 0.22) | (2.31, 0.02) | (3.88, 0a) | (1.51, 0.14) | (1.19, 0.24) | (2.41, 0.02) | (2.7, 0.01) | (0.47, 0.64) |
ap<0.01
Table 4: Effects of age, behavioral scores and head motion on intra-ICN ALFFs for normal controls.
▪ Altered development of intra-ICN ALFFs between ADHD group and normal control group
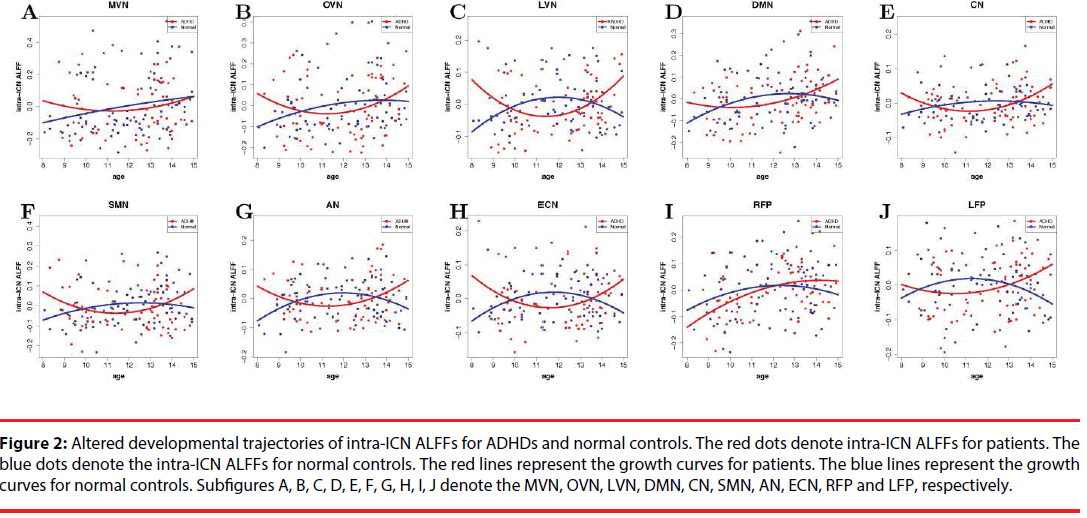
Table 5 and Figure 2 show the altered development of intra-ICN ALFFs between ADHD group and normal control group. Compared to normal control group, the U shapes of the growth curves for certain networks are altered in ADHD group. The developmental curves for lateral visual network, auditory network, and executive control network are significantly altered between the two groups. Table 6 shows the turning points of developmental brains for ADHD group and normal control group. Based on intra-ICN ALFFs within lateral visual network, auditory network, and executive control network, the turning points of brain developments might be 11-12 years old for ADHD.
| Names | (T, p) |
|---|---|
| ICN1 | (1.49, 0.14) |
| ICN2 | (1.68, 0.09) |
| ICN3 | (2.1, 0.03) |
| ICN4 | (0.65, 0.52) |
| ICN5 | (1.12, 0.28) |
| ICN6 | (1.88, 0.06) |
| ICN7 | (2.02, 0.04) |
| ICN8 | (2.45, 0.02) |
| ICN9 | (-1.28, 0.21) |
| ICN10 | (0.24, 0.81) |
Table 5: Altered growth curves between ADHDs and normal controls.
Figure 2: Altered developmental trajectories of intra-ICN ALFFs for ADHDs and normal controls. The red dots denote intra-ICN ALFFs for patients. The blue dots denote the intra-ICN ALFFs for normal controls. The red lines represent the growth curves for patients. The blue lines represent the growth curves for normal controls. Subfigures A, B, C, D, E, F, G, H, I, J denote the MVN, OVN, LVN, DMN, CN, SMN, AN, ECN, RFP and LFP, respectively.
| Names | ADHDs | Normal controls |
|---|---|---|
| ICN1 | 11.2 | 15 |
| ICN2 | 11.2 | 13.8 |
| ICN3 | 11.4 | 12 |
| ICN4 | 10.1 | 12.7 |
| ICN5 | 11.1 | 12.4 |
| ICN6 | 11.4 | 12.6 |
| ICN7 | 11.3 | 12 |
| ICN8 | 11.6 | 11.8 |
| ICN9 | 8 | 12.3 |
| ICN10 | 10.5 | 11.3 |
Table 6: Turning points for developmental brains based on intra-ICN ALFFs.
Discussions
In this paper, we investigated the developmental curves of large-scale networks for school-age boys with ADHD by analyzing the ALFFs within intrinsic connectivity networks (ICNs). To our knowledge, this was the first study attempted to model the growth curves of ICNs for ADHD’s brain. The results demonstrated that the maturity of functional connectivity within ICNs might be delayed in children with ADHD, suggesting the intra-ICN ALFFs could be beneficial to investigating the development of human functional connectome.
Patients with ADHD were reported with altered functional connectivity in sensory-related brain regions. Our results found that the developments of intra-ICN ALFFs were altered in lateral visual network, cerebellum, and auditory network. Abnormal structural connectivity between thalamus and sensory-motor cortex was found in patients with ADHD based on DTI tractography [28]. Altered ALFF of left sensory-motor cortex were found in children with ADHD using resting state fMRI [6]. Disrupted functional connectivity between default mode network and visual network was found in children with ADHD [29]. The above studies supported our findings that the development of sensory-related brain network might be lagged in patients with ADHD.
The abnormal developments of intra-ICN ALFFs in executive control network suggested that the executive function might be altered in patients with ADHD. The abnormal executive control network might reflect the dysfunction of action-inhibition as well as emotion-regulation in ADHD [25]. The executive control-related brain regions also exhibited thinning cortical thickness, according to a previous study based on brain morphometry [2]. Overall, the intra-ICN ALFFs in executive control network might play an important role in the developments of ADHD.
The developmental trajectories based on intra- ICN ALFFs exhibited as U-shaped curves for patients with ADHD during childhood. Previous findings suggested that the lagged brain development might explain the vulnerability of the patients’ attention [13]. The linear model based on inter-ICN connectivity also found slow maturation in children with ADHD [12]. Our results provided additional information of brain maturation-related studies. The U-shaped curves indicated the turning points of brain maturation might be 11-12 years old for children with ADHD. Moreover, the U-shaped curves implied that the brain developments of ADHD might be delayed before 11-12 years old, and the growth might be out of control after the pitch age. In summary, the results confirmed that the brain maturation were delayed and then distracted in patient group, and suggested that there might be a pitch point during brain development for patients with ADHD.
One advantage of this study was applying intra- ICN ALFFs to investigate the brain developments for children with ADHD. The intra-ICN ALFFs were univariate features, which could reflect the information flow within ICNs. Moreover, the computation procedure for intra-ICN ALFFs was simple and easy for implementation. Thus, the intra-ICN ALFFs could be potential biomarker to measure the temporal dynamics within ICNs. Another advantage of this study was modeling the growth curves of large-scale networks for ADHD. So far, to the best of our knowledge, this might be the first attempt to investigate the brain developmental trajectories for ADHD based on ICNs. In addition, our study found out the turning points for ADHD group and normal control group. The informative results could provide supplementary evidences for brain developments from different aspects.
One limitation of this study was relatively small size of research population (80 ADHDs and 77 normal controls). To remove the effects of scan parameters, gender and clinical scales, we only considered a cohort of boys with ADHD and a cohort of age-matched normal controls in this prospective study. Although the research population is sufficient for a pilot study, we should extend this study on multi-sites datasets with different subtypes, medication status, scan parameters, gender and clinical scales in future study. Another limitation was the limited frequency bands for intra-ICN ALFFs (0.01- 0.1Hz). In future study, we should consider wavelets transform or split the intra-ICN ALFFs into different frequency bands [30]. In addition, other univariate metrics (i.e., entropy, Hurst exponent) could also be considered and compared as intra-ICN complexity in future study [31,32].
Conclusion
In conclusion, intra-ICN ALFFs were applied to analyze the development of ICNs for children with ADHD. The abnormal developments of large-scale network were found in sensory and execution-related ICNs for patients group. The results suggested that the intra-ICN ALFFs could be supplemental biomarkers for neuroimaging studies.
Acknowledgments
This work is supported in part by the grants from National Natural Science Foundation of China (61271063), 973 Program (2013CB329502), National Distinguished Young Research Scientist Award (60788101). This research is partly supported by the Science Foundation from Health Commission of Zhejiang Province, 2013RCA001. We want to thank the ADHD-200 Consortium for sharing resting fMRI datasets. We also want to thank for the two anonymous reviewers for their insightful helps.
References
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions.Biol. Psychiatry57(11), 1273-1284(2005).
- Makris N, Biederman J, Valera EM,et al.Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder.Cereb. cortex17(6), 1364-1375(2007).
- Qiu A, Crocetti D, Adler M,et al.Basal ganglia volume and shape in children with attention deficit hyperactivity disorder.Am. J. Psychiatry166(1), 74-82(2009).
- de Zeeuw P, Mandl RC, Hulshoff Pol HE,et al.Decreased frontostriatal microstructural organization in attention deficit/hyperactivity disorder.Hum. Brain. Mapp33(8), 1941-1951(2012).
- Wang X, Jiao Y, Tang T,et al.Altered regional homogeneity patterns in adults with attention-deficit hyperactivity disorder.Eur. J. Radiol82(9), 1552-1557(2013).
- Zang YF, He Y, Zhu CZ,et al.Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain. Dev29(2), 83-91(2007).
- Castellanos FX, Margulies DS, Kelly C, et al.Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry63(3), 332-337(2008).
- Uddin LQ, Kelly AM, Biswal BB,et al.Network homogeneity reveals decreased integrity of default-mode network in ADHD.J. Neurosci. Methods169(1), 249-254(2008).
- Mackie S, Shaw P, Lenroot R, et al.Cerebellar development and clinical outcome in attention deficit hyperactivity disorder.Am. J. Psychiatry164(4), 647-655(2007).
- Shaw P, Malek M, Watson B,et al.Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder.Biol. Psychiatry72(3), 191-197(2012).
- de Zeeuw P, Schnack HG, van Belle J, et al.Differential brain development with low and high IQ in attention-deficit/hyperactivity disorder. PLoS. one7(4), e35770(2012).
- Choi J, Jeong B, Lee SW,et al.Aberrant development of functional connectivity among resting state-related functional networks in medication-naïve ADHD children.PLoS. one8(12), e83516(2013).
- Sripada CS, Kessler D, Angstadt M. Lag in maturation of the brain’s intrinsic functional architecture in attention-deficit / hyperactivity disorder.Proc. Natl. Acad. Sci. U S A111(39), 14259-14264(2014).
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model.Trends. Cogn. Sci 16(1), 17-26(2012).
- Hoekzema E, Carmona S, Ramos-Quiroga JA, et al.An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum. Brain. Mapp35(4), 1261-1272(2014).
- Wang X, Jiao Y, Tang T, et al.Investigating univariate temporal patterns for intrinsic connectivity networks based on complexity and low-frequency oscillation: a test-retest reliability study.Neuroscience 254(1), 404-426(2013).
- McDonough IM, Nashiro K. Network complexity as a measure of information processing across resting-state networks: evidence from the Human Connectome Project.Front. Hum. Neurosci8(1), 409 (2014).
- http://fcon_1000.projects.nitrc.org/indi/adhd200/
- Milham MP, Fair D, Mennes M, et al.The ADHD-200 Consortium: A Model to Advance the Translational Potential of Neuroimaging in Clinical Neuroscience.Front. Syst. Neurosci6(1), 62 (2012).
- www.fmrib.ox.ac.uk/fsl
- afni.nimh.nih.gov
- Biswal BB, Mennes M, Zuo XN, et al.Toward discovery science of human brain function.Proc. Natl. Acad. Sci. U S A 107(10), 4734-4739 (2010).
- Wang XH, Li L. Altered temporal features of intrinsic connectivity networks in boys with combined type of attention deficit hyperactivity disorder.Eur. J. Radiol84(5), 947-954(2015).
- http://brainmap.org/tools.html
- Smith SM, Fox PT, Miller KL,et al.Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U S A 106(31), 13040-13045(2009).
- Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion.NeuroImage 59(3), 2142-2154(2012).
- http://www.ugrad.stat.ubc.ca/R/library/statmod/html/growthcurve.html
- Xia S, Li X, Kimball AE, et al.Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry. Res204(2-3), 161-167(2012).
- Sripada C, Kessler D, Fang Y,et al.Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder.Hum. Brain. Mapp35(9), 4693-4705(2014).
- Zuo XN, Di Martino A, Kelly C, et al.Castellanos, B.B. Biswal, M.P. Milham, The oscillating brain: complex and reliable.Neuroimage 49(2), 1432-1445(2010).
- Lei X, Zhao Z, Chen H. Extraversion is encoded by scale-free dynamics of default mode network, Neuroimage 74(1), 52-57(2013).
- Wei M, Qin J, Yan R, et al.Identifying major depressive disorder using Hurst exponent of resting-state brain networks.Psychiatry. Res214(3), 306-312(2013).